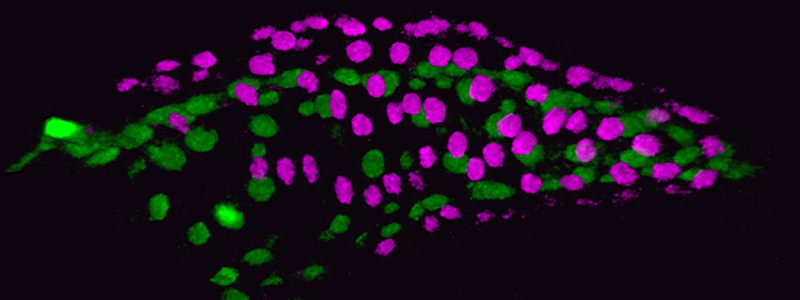
Developing Zebrafish Heart

Carnivorous flowering plant

Wildflower diversity in a restored open oak woodland

Diversity of wild mushrooms of north Mississippi

Savannah meadowbeauty (Rhexia alifanus)

Young shark being studied in the Gulf of Mexico

Tarantula photographed in Arizona
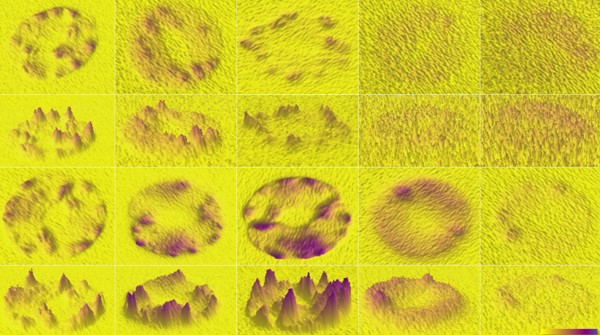
Visualization of Reactive Oxygen Species in Guard Cells
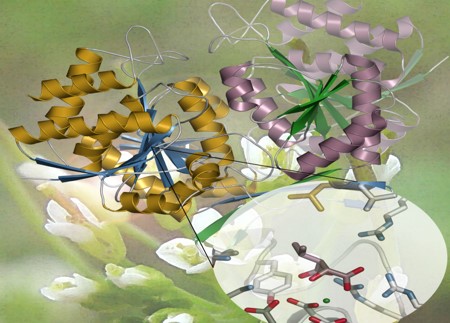
The first crystallized plant isopropylmalate dehydrogenase