| ****************************** UM Affiliated ****************************** |
40. Prakash, D.; Sony, S.; Chakraborty, S.* “Tuning the Function of De Novo Designed Artificial Cu Proteins by Modulating Reorganization Energies"; Methods Enzymol. (special issue on Artificial Metalloproteins, Ed. Lisa Olshansky), 2025, 723 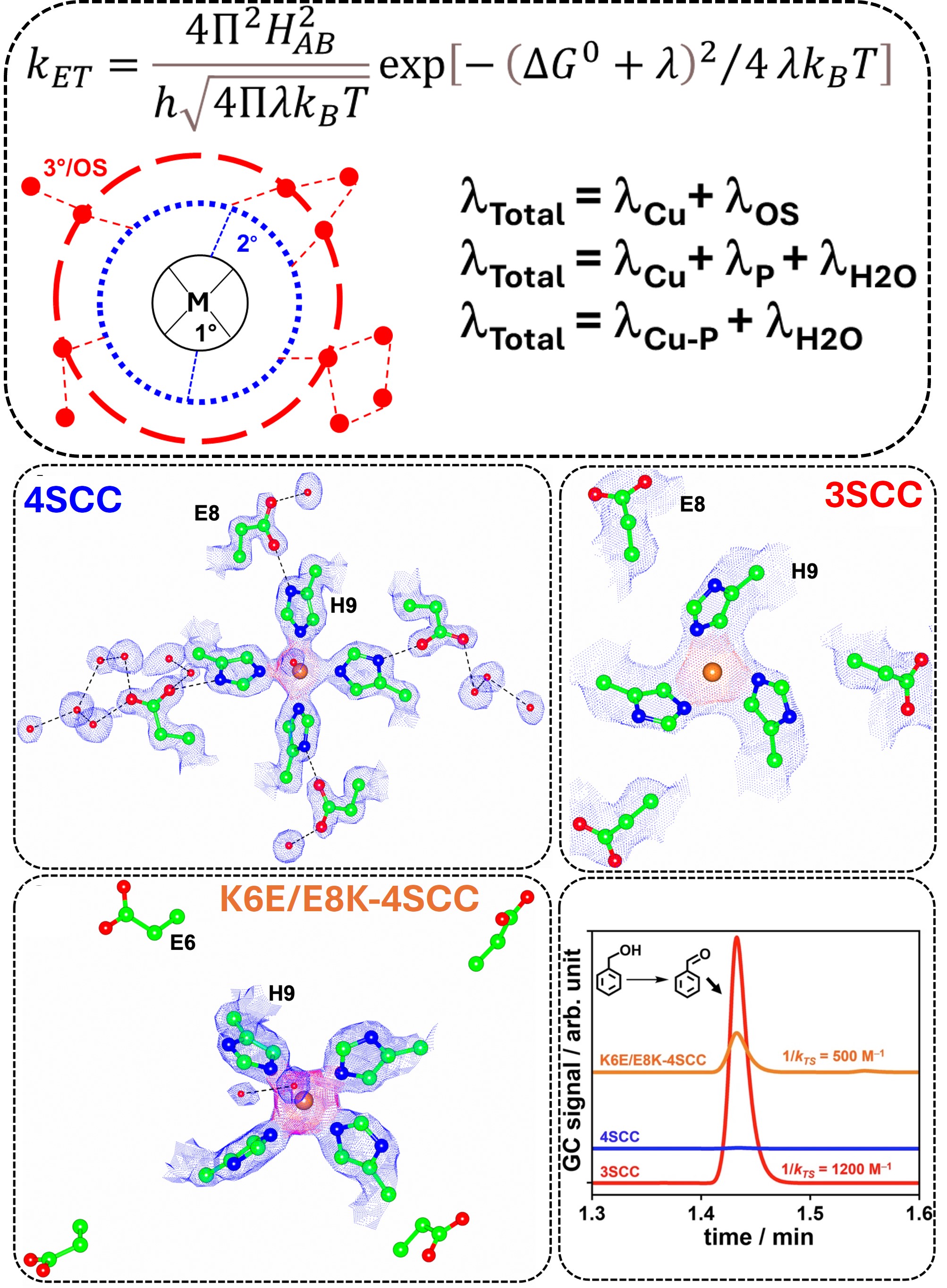 |
39. Prakash, D.;% Wu, Y.;% Misra, S. K.; Sampath, N.; Wang, B.*; Chakraborty, S.* “Mechanism of Oxidative C-H Bond Activation by De Novo Designed Artificial Cu Metalloenzymes Using H2O2 and O2"; Chem. Eur. J. 2025, 0, e01245 (%equal contributing author) 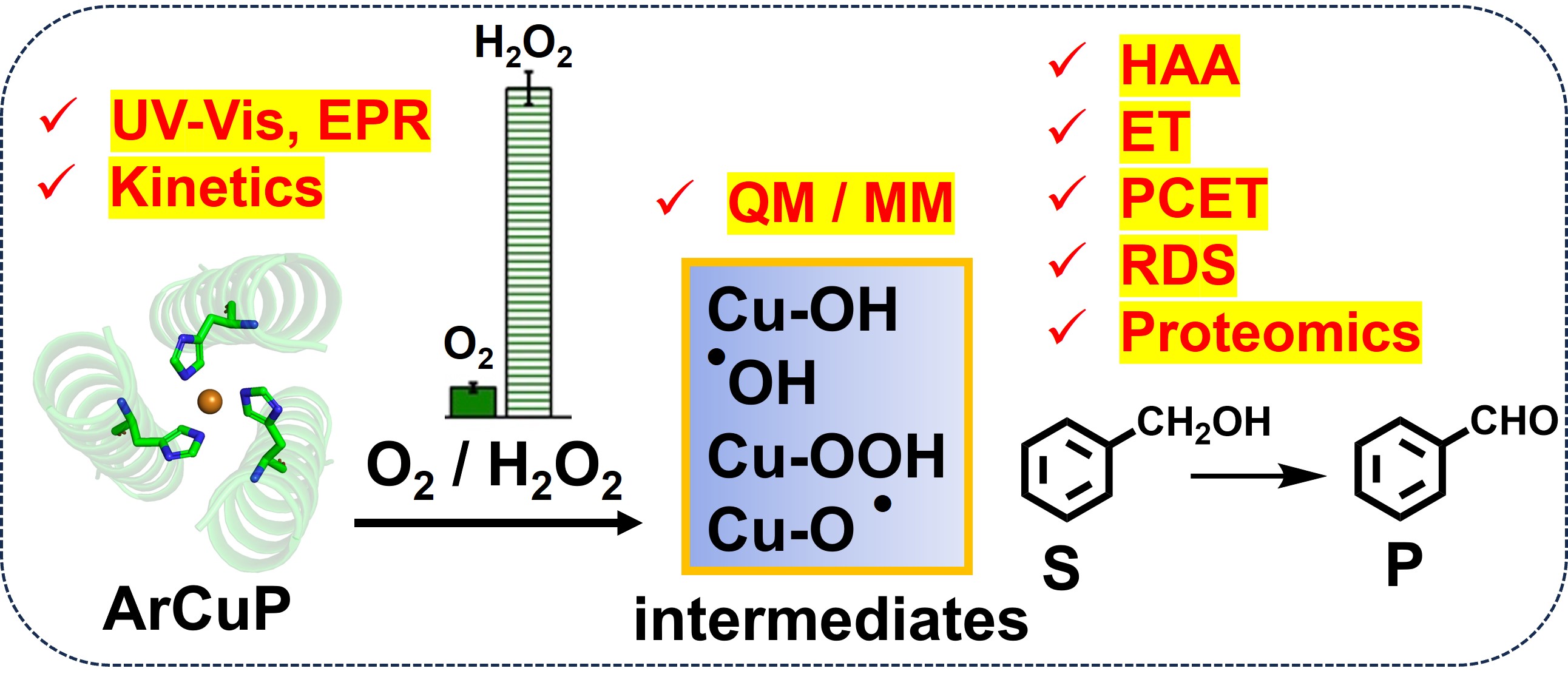 |
38. Prakash, D.; Mitra, S.; Sony, S.; Murphy, M.; Andi, B.; Ashley, L.; Prasad, P.; Chakraborty, S.* “Controlling outer-sphere solvent reorganization energy to turn on or off the function of artificial metalloenzymes"; Nat. Commun. 2025, 16, 3048 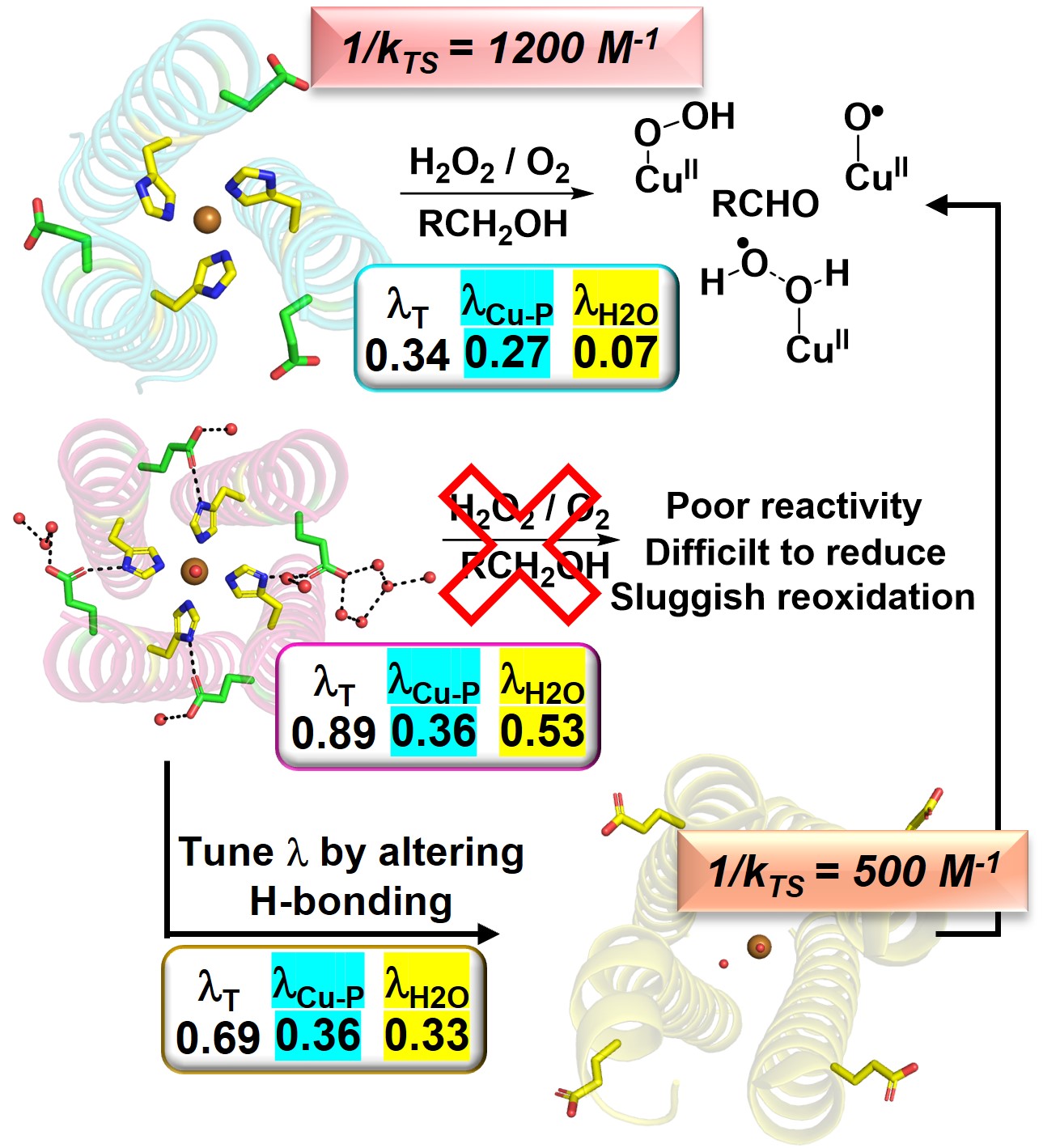 |
37. Parambath, S. M.; Prakash, D.; Swetman, W.; Surakanti, A.; Chakraborty, S.* “Converting a Cysteine-Rich Natively Noncatalytic Protein to an Artificial Hydrogenase"; Chem.Comm. 2023, 59, 13325-13328 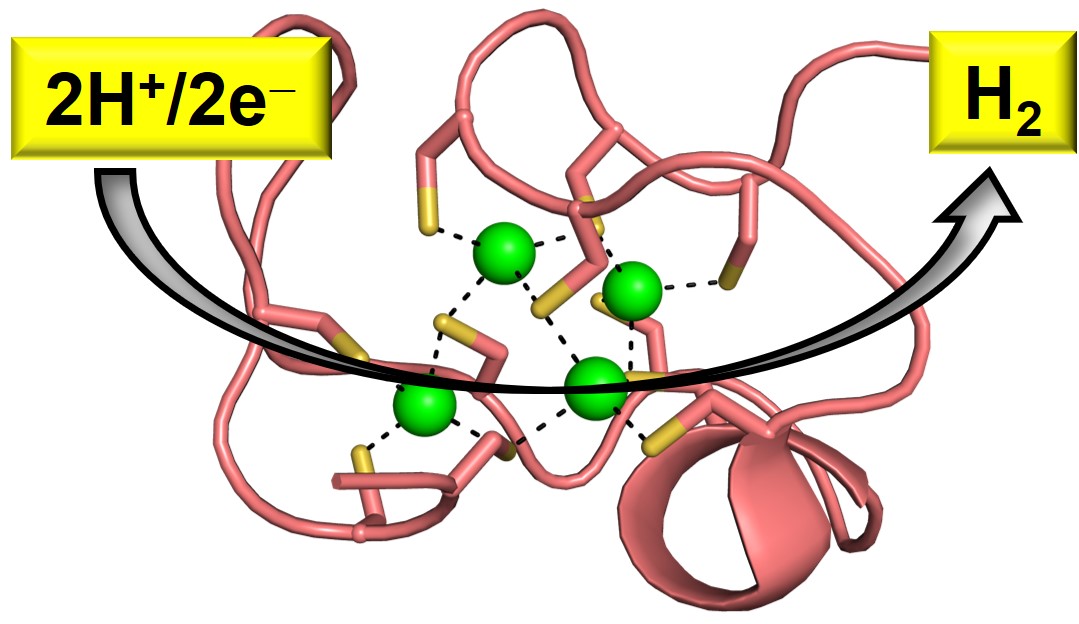 |
36. Selvan, D.; Chakraborty, S.* “A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase"; Int. J. Mol. Sci. 2023, 24, 10317 This article is part of a Special Issue (Eds. Drs. Angela Lombardi, Flavia Nastri, Linda Leone) “Natural, Designed and Engineered Metalloenzymes: Structure, Catalytic Mechanisms and Applications"; 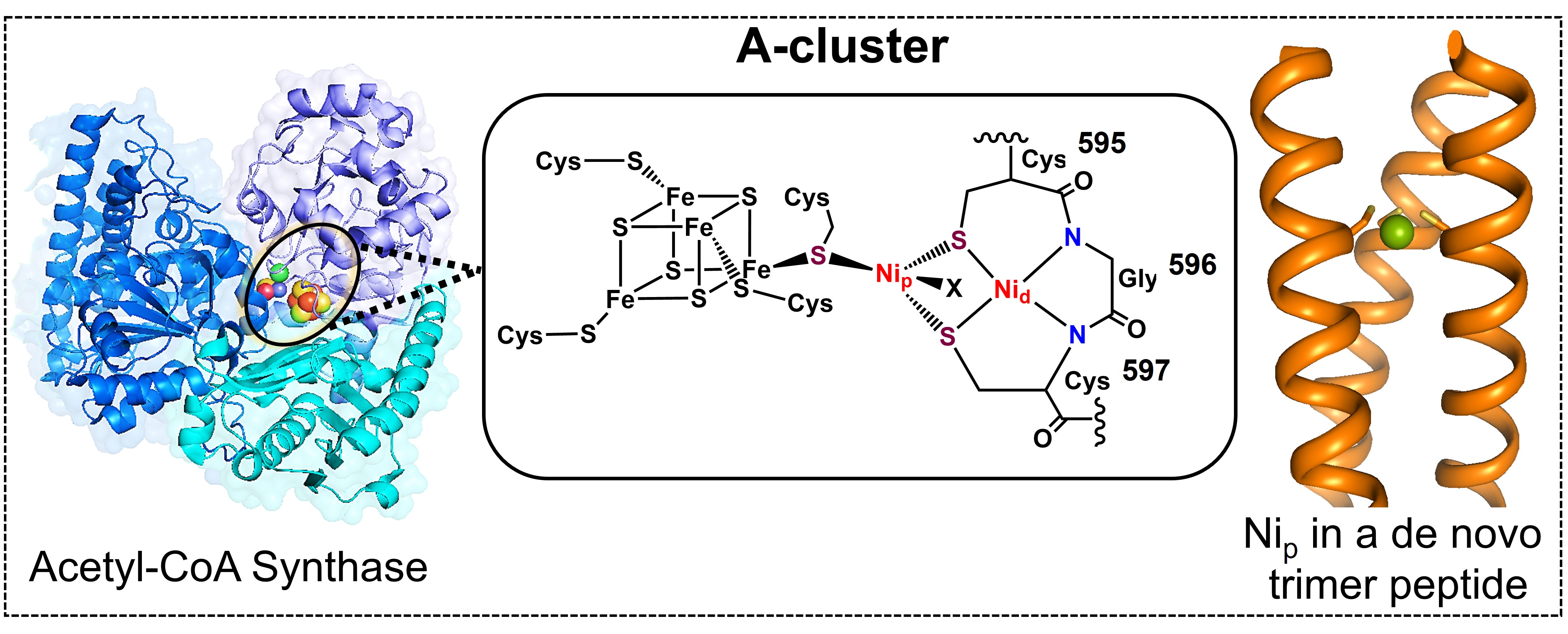 |
35. Prasad, P.; Hunt, L. A.; Pall, A. E.; Ranasinghe, M.; Williams, A. E.; Stemmler, T. L.; Demeler, B.; Hammer, N. I.; Chakraborty, S.* “Photocatalytic Hydrogen Evolution by a De Novo Designed Metalloprotein that Undergoes Ni-Mediated Oligomerization Shift"; Chem. Eur. J. 2023, 29, e202202902 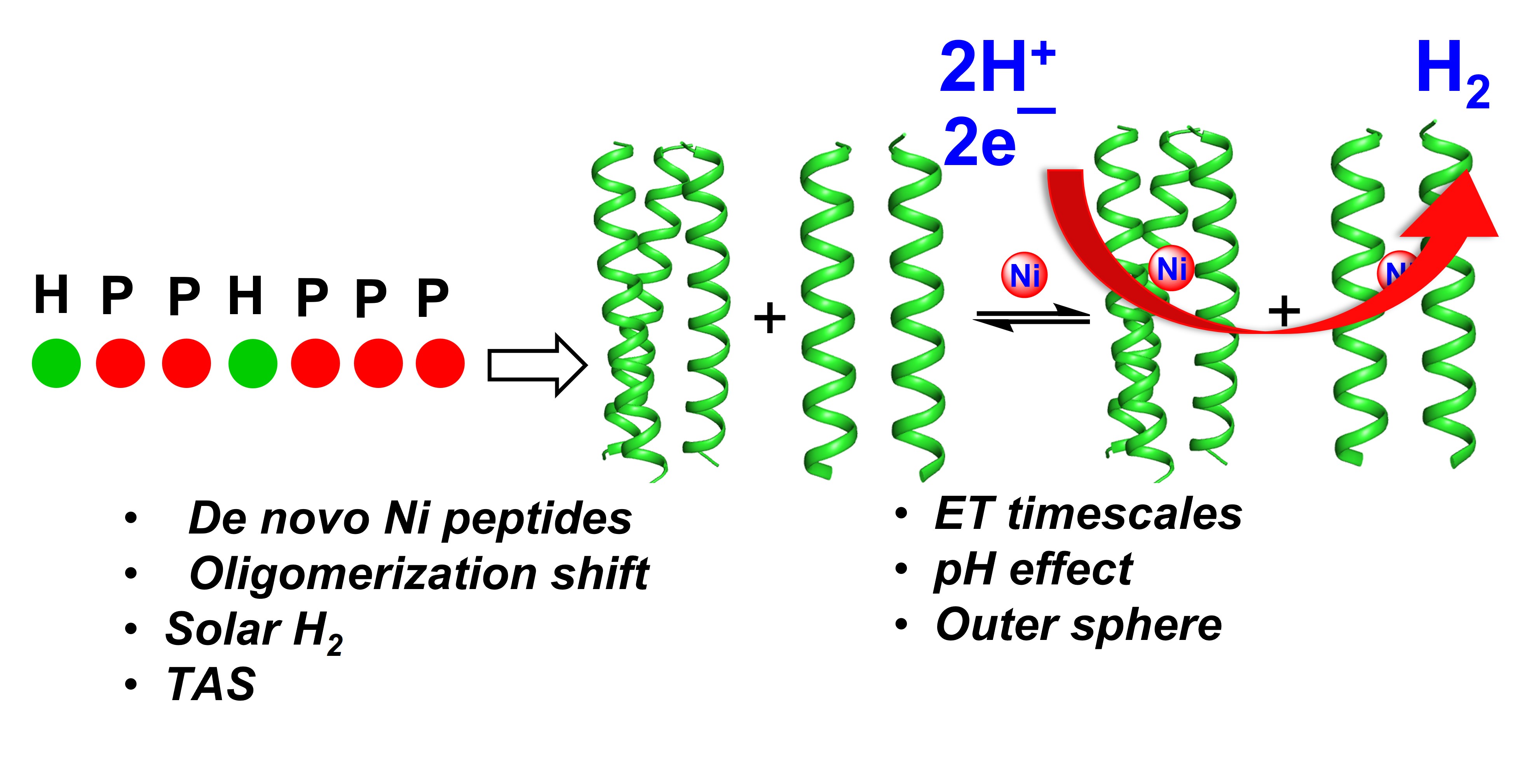 |
34. Prakash, D.; Mitra, S.; Murphy, M.; Chakraborty, S.* “Oxidation and Peroxygenation of C-H Bonds by Artificial Cu Peptides (ArCuPs): Improved Catalysis via Selective Outer Sphere Modifications"; ACS Catal. 2022, 12, 8341-8351 “Pre-print"; ChemRxiv 2022 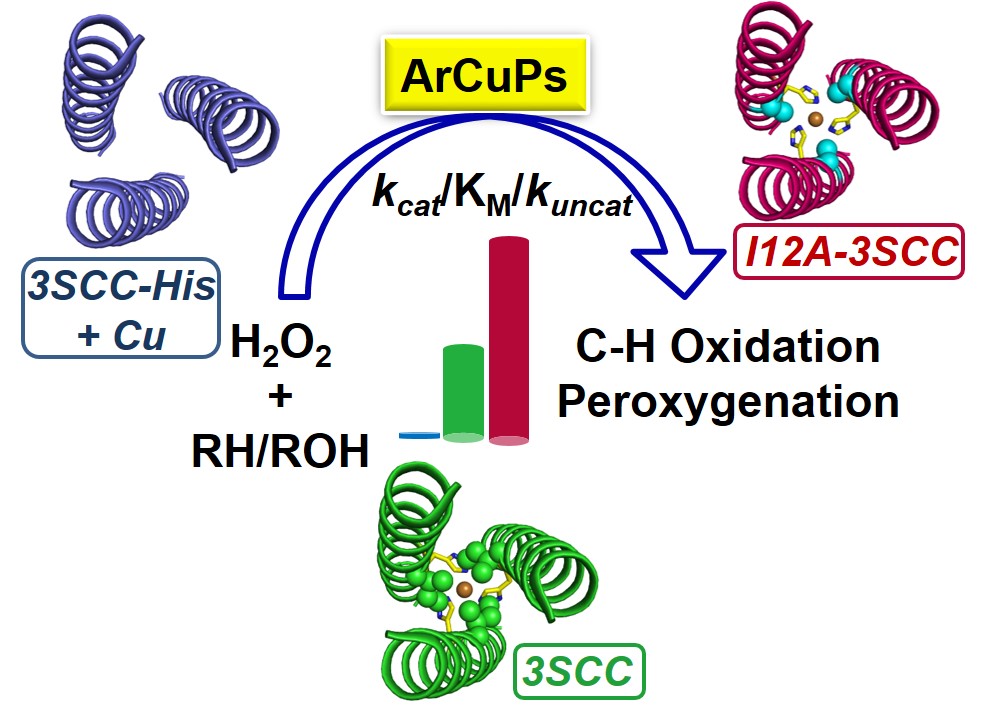 |
33. Mitra, S.; Talukdar, K.; Prasad, P.; Misra, S. K.; Khan, S.; Sharp, J.; Jurss, J. W.; Chakraborty, S.* “ Rational Design of a Cu Chelator that Mitigates Cu-Induced ROS Production by Amyloid Beta"; ChemBioChem 2022, 23, e202100485 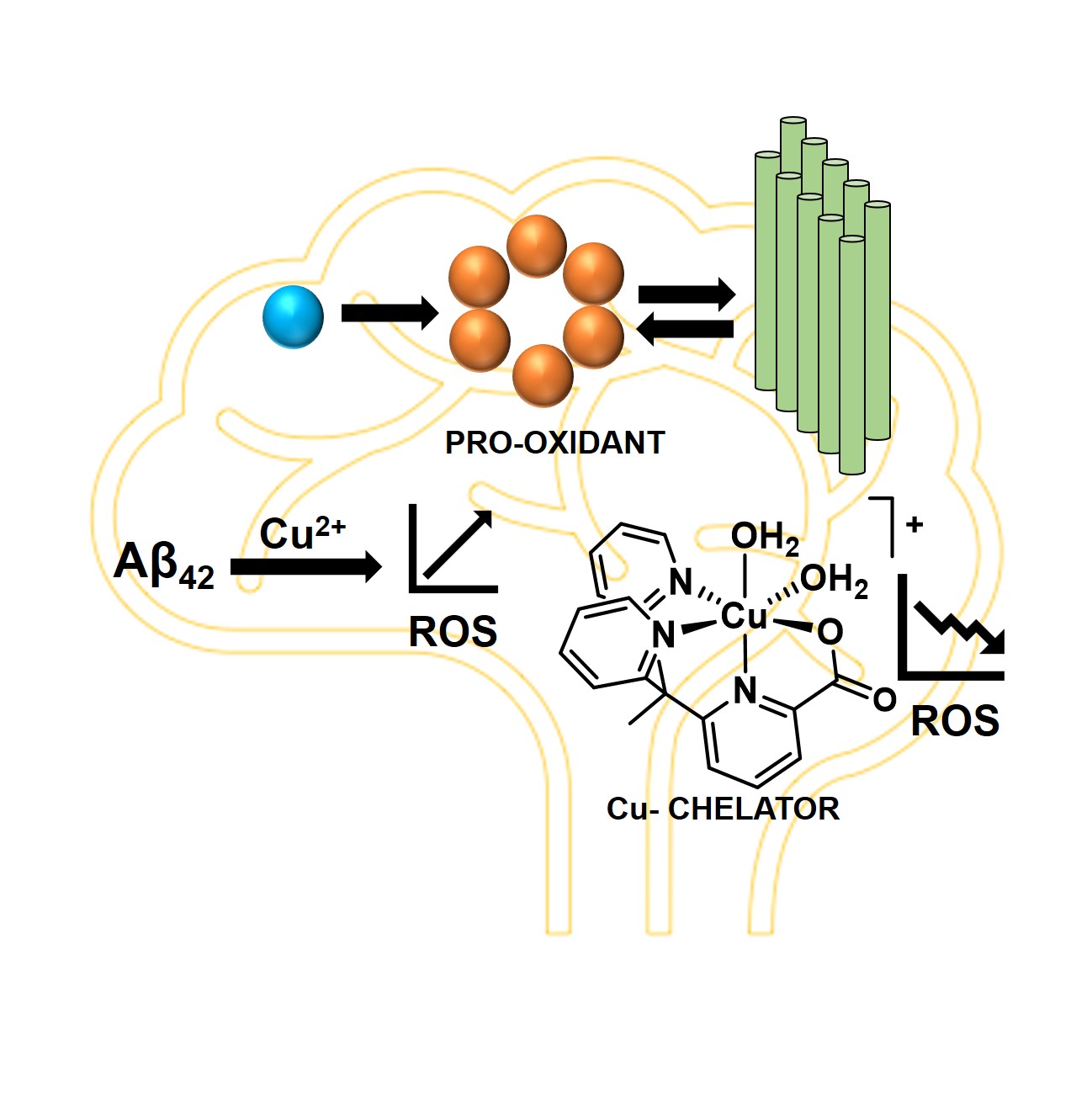 |
32. Mitra, S.; Prakash, D.; Rajabimoghadam, K.; Wawrzak, Z.; Prasad, P.; Wu, T.; Misra, S.; Sharp, J. S.; Garcia-Bosch, I.; Chakraborty, S.* “De Novo Design of a Self-Assembled Artificial Copper Peptide that Activates and Reduces Peroxide"; ACS Catal. 2021, 11, 10267-10278 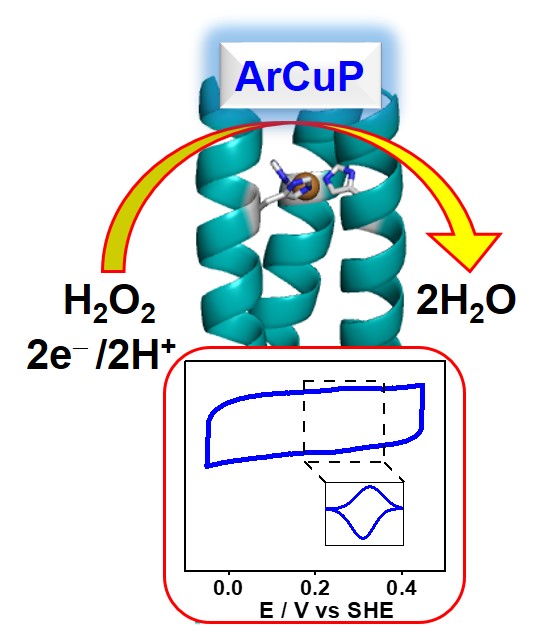 |
31. Parambath, S. M.; Williams, A. E.; Hunt, L. A.; Selvan, D.; Hammer, N. I.; Chakraborty, S.* “A De Novo Designed Artificial Metallopeptide Hydrogenase: Insights into Photochemical Processes and the Role of Protonated Cys; ChemSusChem 2021, 14, 2237-2246 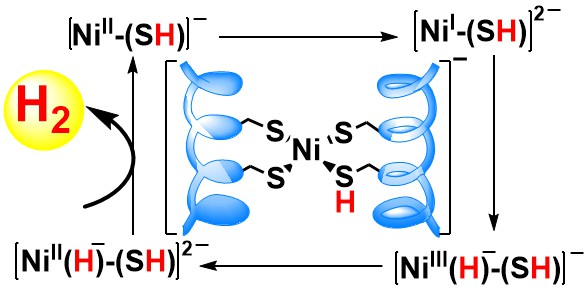 |
30. Prasad, P; Selvan, D.; Chakraborty, S.* “Biosynthetic Approaches Towards the Design of Artificial Hydrogen Evolution Catalysts; Chem. Eur. J. 2020, 26, 12494-12509  |
29. Nazemi, Z; Prasad, P.; Chakraborty, S.* “Kinetics of Oxygen Reduction by a Beta Barrel Heme Protein on Hybrid Bioelectrodes; ChemElectroChem. 2020, 7, 1029-1037 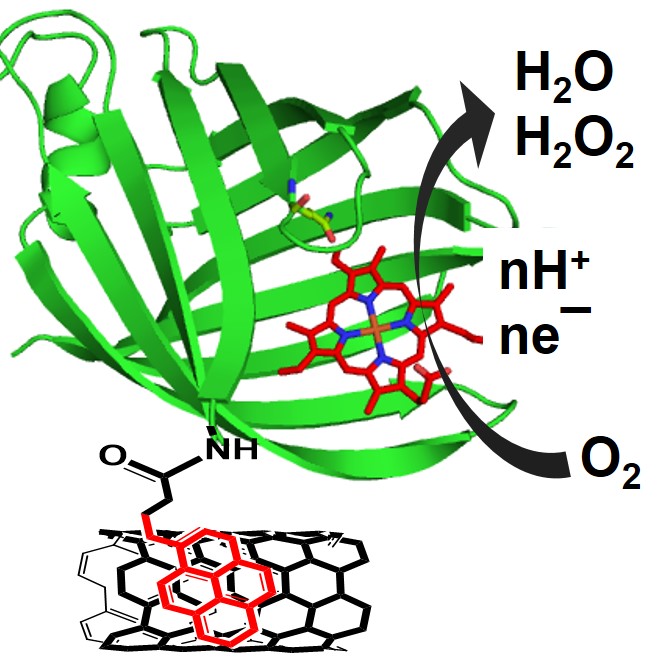 |
28. Selvan, D.; Shi, Y.; Prasad, P.; Crane, S.; Zhang, Y.*; Chakraborty, S.* “The Oxygen Reactivity of an Artificial Hydrogenase Designed in a Reengineered Copper Srotage Protein” Dalton Trans. 2020, 49, 1928-1934 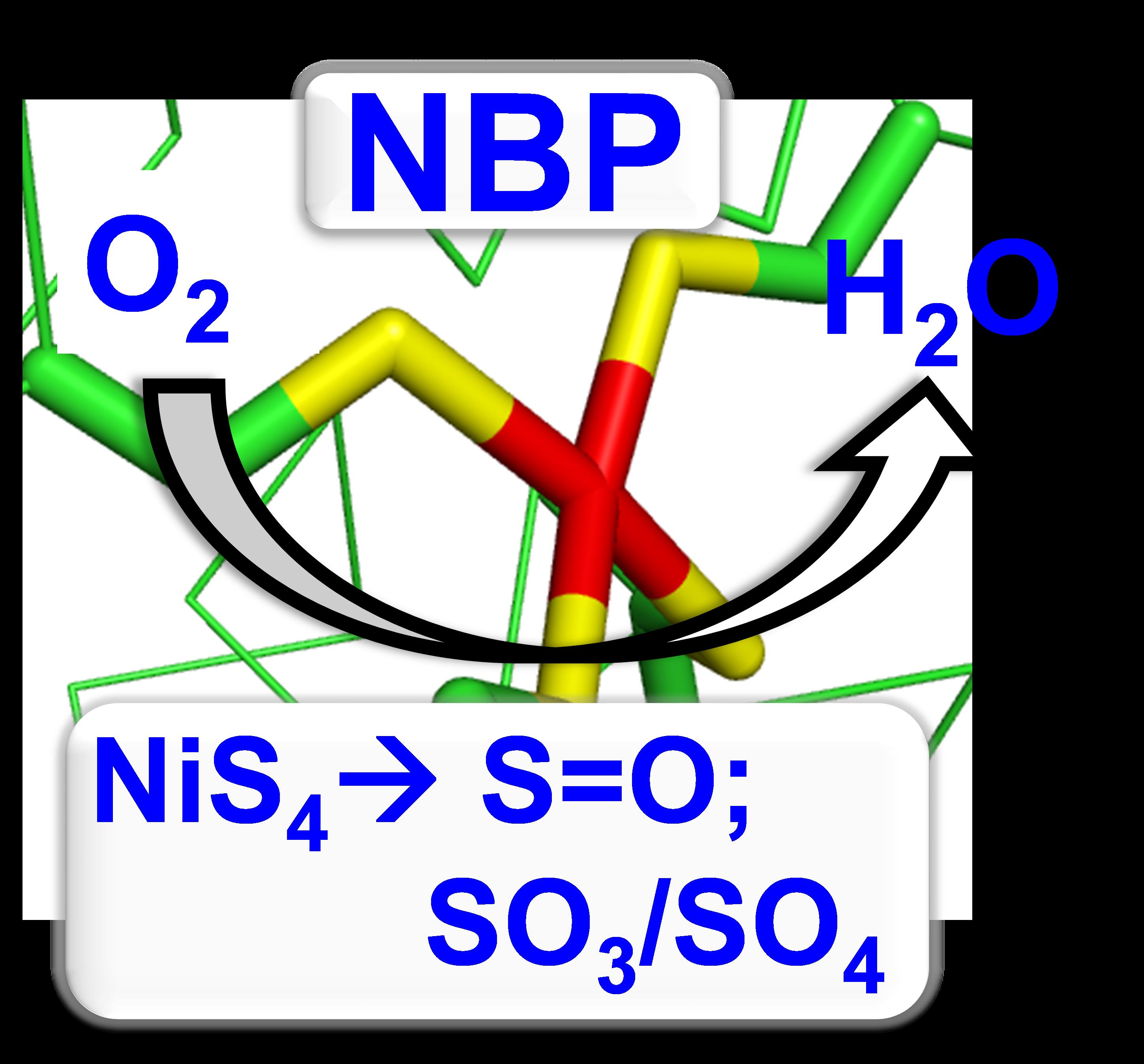 |
27. Selvan, D.; Prasad, P.; Farquhar, E.; Shi, Y.; Crane S.; Zhang, Y.; Chakraborty, S.* “Redesign of a Copper Storage Protein into an Artificial Hydrogenase” ACS Catal. 2019, 9, 5847-5859 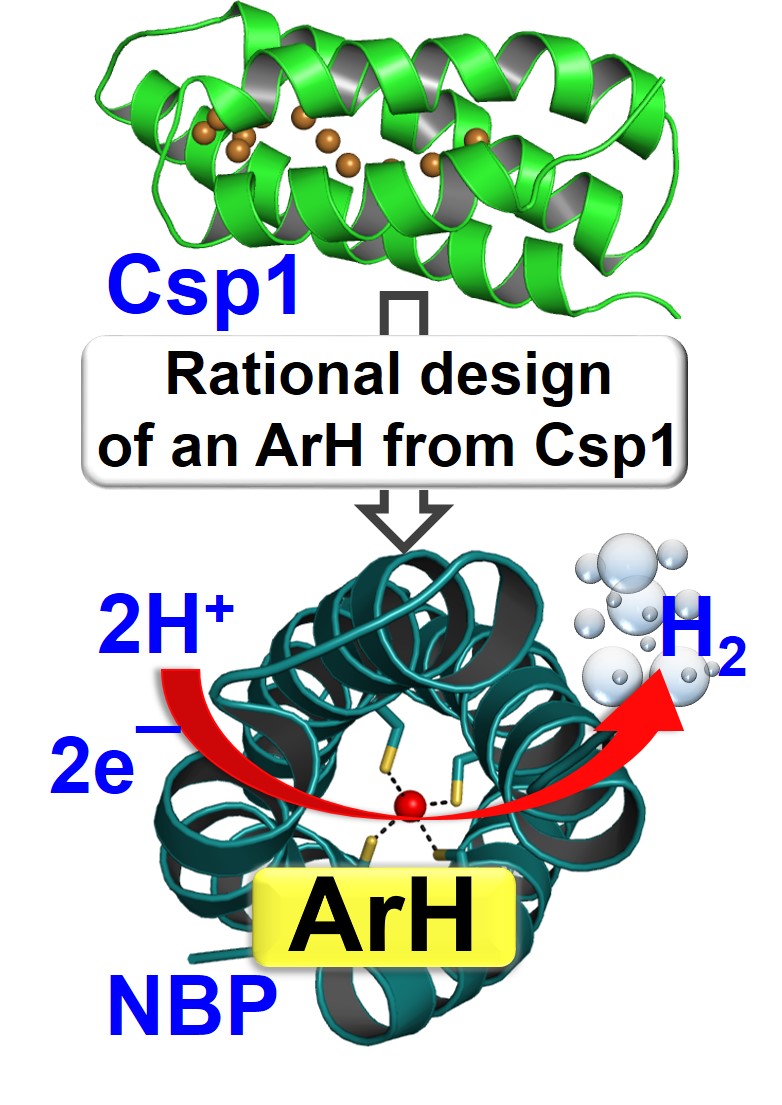 |
26. Selvan, D.; Prasad, P.; Crane, S.; Abuhagr, A.; Artyushkova, K.; Guda, R.; Chakraborty, S.* “Intrinsically Fluorescent Gold Nanoclusters Stabilized within a Copper Storage Protein that Follow Irving-Williams Trend in Metal Ion Sensing” Analyst 2019, 144, 3949-3958  |
25. Sumner, L.; Sakthivel, N. A.; Schrock, H.; Artyushkova, K.; Dass, A.*; Chakraborty, S.* “Electrocatalytic Oxygen Reduction Activities of Thiol Protected Nanomolecules Ranging in Size from Au28(SR)20 to Au279(SR)84” J.Phys. Chem. C. 2018, 122, 24809-24817 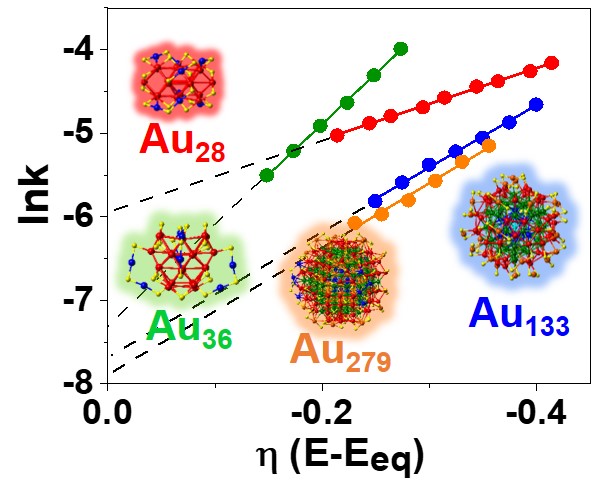 |
24. Chen, Y.; Phipps, M. L.; Werner, J. H.; Chakraborty, S.*; Martinez, J. S.* “DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications” Acc. Chem. Res. 2018, 51, 2756-2763  |
23. Mitra, S.; Pallavi, P.; Chakraborty, S.* “A Unified View of Assessing the Pro-Oxidant vs Antioxidant Nature of Amyloid Beta Conformers” ChemBioChem. 2018, 19, 2360-2371 “Cover Feature” |
22. Jones, T.; Sumner, L.; Ramakrishna, G.; Bin Hatshan, M.; Abuhagr, A; Chakraborty, S.*; Dass, A.* “Bulky t-Butyl Thiolated Gold Nanomolecular Series: Synthesis, Characterization, Optical Properties, and Electrocatalysis” J. Phys. Chem. C. 2018, 122, 17726-17737 “Cover Feature”  |
21. Reed, J.; Shi, Y.; Zhu, Q.; Chakraborty, S.; Mirts, E.; Petrik, I.; Bhagi-Damodaran, A.; Ross, M.; Moenne-Loccoz, P.*; Zhang, Y.*; Lu, Y.* “Manganese and Cobalt in the Nonheme Metal-binding Site of a Biosynthetic Model of Heme-Copper Oxidase Superfamily Confer Oxidase Activity through Redox-inactive Mechanism” J. Am. Chem. Soc. 2017, 139, 12209-12218 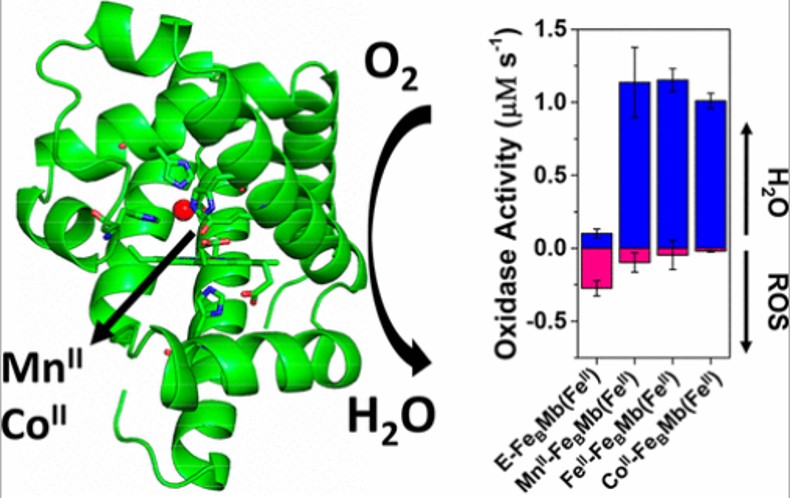 |
20. Chakraborty, S.; Pallada, S.; Pedersen, J. T.; Jancso, A.; Correia, J. G.; Hemmingsen, L.*; “Nanosecond Dynamics at Protein Metal Sites: An Application of Perturbed Angular Correlation (PAC) of g-Rays Spectroscopy” Acc. Chem. Res. 2017, 50, 2225-2232 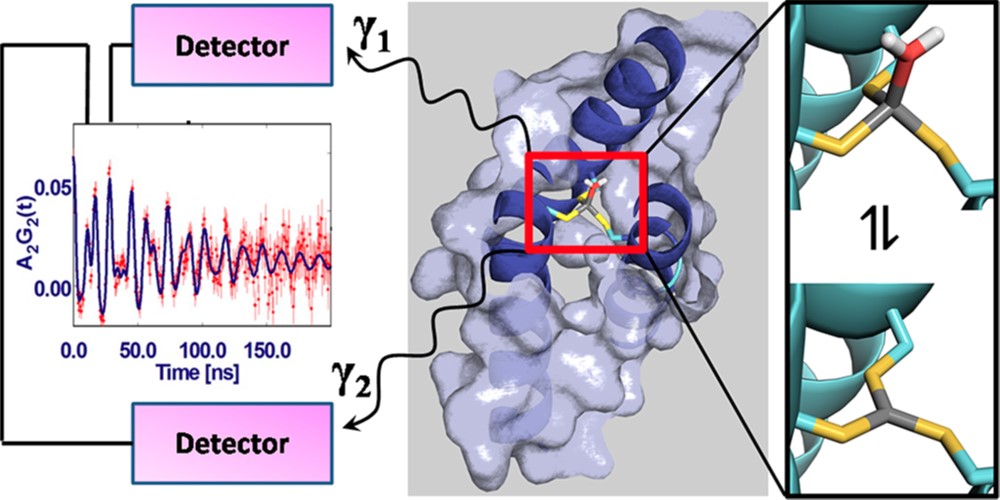 |
19. Chakraborty, S.*; Rocha, R. C.; Desireddy, A.; Artyushkova, K.; Perry, A.T.; Sanchez, T.; Atanassov, P.; Martinez, J. S.* “Gold Nanocluster Formation Using Morpholino Oligomer as Template and Assembly Agent Within Hybrid Bio-nanomaterials” RSC Adv. 2016, 6, 90624-90630 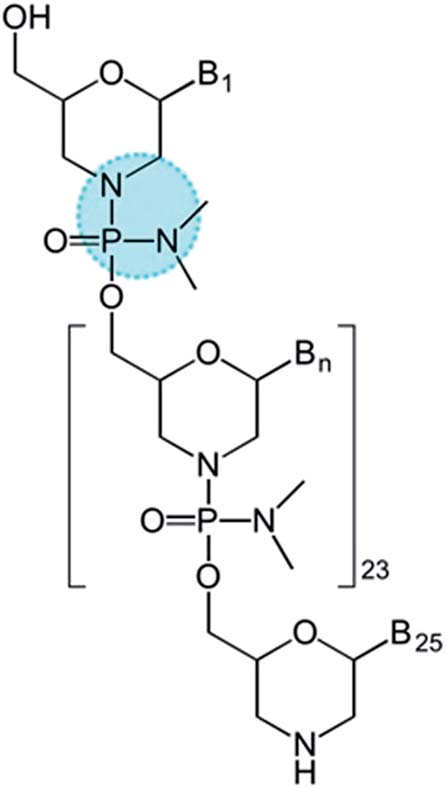 |
| ------------------------------------------------------------Prior to UM------------------------------------------------------------ |
| 18. Fazelinia, H.; Balog, E. R. M.; Desireddy, A.; Chakraborty, S.; Sheehan, C. J.; Strauss, C. E. M.; Martinez, J. S. “Genetically Engineered Elastomeric Polymer Network through Protein Zipper Assembly” Chemistry Select 2017, 2, 5005-5012. |
17. Stachura, M.; Chakraborty, S.; Gottberg, A.; Ruckthong, L.; Pecoraro, V. L.; Hemmingsen, L. “Direct Observation of Nanosecond Water Exchange Dynamics at a Protein Metal Site” J. Am. Chem. Soc. 2017, 139, 79-82. |
16. Bhagi-Damodaran, A.; Michael, M. A.; Zhu, Q.; Reed, J.; Sandoval, B.A.; Mirts, E.; Chakraborty, S.; Moenne-Loccoz, P.; Zhang, Y.; Lu, Y. “Why Copper is Preferred Over Iron for Oxygen Activation and Reduction in Heme-Copper Oxidases” Nat. Chem. 2017, 9, 257-263. |
| 15. Matsumura, H.; Chakraborty, S.; Reed, J.; Lu, Y.; Moenne-Loccoz, P. “Effect of Outer-Sphere Side Chain Substitutions on the Fate of the trans Iron-Nitrosyl Dimer in Heme/Nonheme Engineered Myoglobins (FeB Mbs): Insights into the Mechanism of Denitrifying NO Reductases” Biochemistry, 2016, 55, 2091-2099 |
14.Chakraborty, S.; Babanova. S.; Rocha, R. C.; Desireddy, A.; Artyushkova, K.; Boncella, A. E.; Atanassov, P.; Martinez, J. S. “A Hybrid DNA-Templated Gold Nanocluster For Enhanced Enzymatic Reduction of Oxygen” J. Am. Chem. Soc. 2015, 137, 11678-11687 (highlighted in Science Daily, EurekAlert!, Los Alamos Daily Post, Santa Fe New Mexican, Nanotechnology News, Phys.Org, Clean Technica, Fuel Cell Works, Biofuels International) |
| 13.Chakraborty, S.; Wilson, T. D.; Polen, M.; Yu, Y.; Nilges, M. J.; Lu, Y. “Binuclear CuA Formation in Biosynthetic Models of CuA in Azurin Proceeds via a Novel Cu(Cys)2 His Mononuclear Copper Intermediate” Biochemistry, 2015, 54, 6071-6081 |
| 12.Chakraborty, S.; Reed, J.; Sage, J. T.; Branagan, N. C.; Petrik, I. D.; Hu, M. Y.; Zhao, J.; Alp, E. E.; Schulz, C. E.; Lu, Y. “Recent Advances in Biosynthetic Models of Nitric Oxide Reductases and Insights Gained from Nuclear Resonance Vibrational and Other Spectroscopic Studies” Inorg. Chem. 2015, 54, 9317-9329 |
| 11.Chakraborty, S.; Hosseinzadeh, P.; Lu, Y. “Metalloprotein Design and Engineering” Encyclopedia of Inorganic and Bioinorganic Chemistry, R.A. Scott, ed.; John Wiley and Sons, Ltd.: Chichester; 2014, pp. 1-51 |
| 10.Chakraborty, S.; Reed, J.; Ross, M.; Nilges, M. J.; Petrik, I. D.; Ghosh, S.; Hammes-Schiffer, S.; Sage, J. T.; Zhang, Y.; Schulz, C. E.; Lu, Y. “Spectroscopic and Computational Study of a Non-heme Iron-Nitrosyl Center in a Biosynthetic Model of Nitric Oxide Reductase” Angew. Chem. Int. Ed. 2014, 56, 2417-2421 |
| 9. Matsumura, H.; Hayashi, T.; Chakraborty, S.; Lu, Y.; Moenne-Loccoz, P. “The Production of Nitrous Oxide by the Heme/Non-heme Diiron Center of Engineered Myoglobins (Fe(B) Mbs) Proceeds Through a trans Iron-Nitrosyl Dimer” J. Am. Chem. Soc., 2014, 136, 2420-2431 |
| 8. Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A. Lu, Y. “Metalloproteins Containing Cytochrome, Iron-sulfur or Copper Redox Centers” Chem. Rev. (special issue on bioinorganic enzymology II, Eds. Solomon, Edward, and Holm, Richard), 2014, 114, 4366-4469 (co-first author) |
| 7. Luczkowski, M.; Zeider, B. A.; Hinz, A. V. H.; Stachura, M.; Chakraborty, S.; Hemmingsen, L.; Huffman, D. L.; Pecoraro, V. L. “Probing the Coordination Environment of the Human Copper Chaperone HAH1: Characterization of a Hg(II)-Bridged Homodimeric Species in Solution” Chem. Eur. J., 2013, 19, 9042-9049 |
| 6. Lu, Y.; Chakraborty, S.; Miner, K. D.; Wilson, T. D.; Mukherjee, A.; Yu, Y.; Liu, J.; Marshall. N. M. “Metalloprotein Design” in Comprehensive Inorganic Chemistry II; Eds. Reedijk, J., Poeppelmeier, K., Elsevier Science, 2013, 3, 565-593 |
| 5.Chakraborty, S.; Iranzo O.; Zuiderweg, E. R. P.; Pecoraro V. L. “Experimental and Theoretical Evaluation of Multisite Cadmium(II) Exchange in Designed Three-Stranded Coiled-Coil Peptides” J. Am. Chem. Soc., 2012, 134, 6191-6203 |
| 4. Chakraborty, S.; Kravitz, J. Y.; Thulstrup, P. W.; Hemmingsen, L.; DeGrado, W.F.; Pecoraro, V. L. “Design of a Three-Helix Bundle Capable of Binding Heavy Metals in a Triscysteine Environment” Angew. Chem. Int. Ed. 2011, 50, 2049-2053. (Highlighted as Inside Cover) |
| 3. Iranzo, O.; Chakraborty, S.; Hemmingsen, L.; Pecoraro, V. L. “Controlling and Fine Tuning the Physical Properties of Two Identical Metal Coordination Sites in De Novo Designed Three Stranded Coiled Coil Peptides” J. Am. Chem. Soc., 2011, 133, 239-251 (co-first author) |
| 2. Chakraborty, S.; Touw, D. S.; Peacock, A. F. A.; Stuckey, J. A.; Pecoraro, V. L. “Structural Comparisons of Apo- and Three Stranded Coiled Coils Clarify Metal Binding Determinants in Thiolate Containing Designed Peptides” J. Am. Chem. Soc., 2010, 132, 13240-13250 |
| 1. Maji, S.; Patra, S.; Chakraborty, S.; Janardanan, D.; Mobin, S. M.; Sunoj, R. B.; Lahiri, G. K. “Valence-State Distribution in the Ruthenium o-Quinonoid Systems [Ru(trpy)-(Cl)(L1)]+ and [Ru(trpy)-(Cl)(L2)]+ (L1 = o-Iminobenzoquinone, L2 = o-Diiminobenzoquinone; trpy = 2,2':6',2''-Terpyridine)” Eur. J. Inorg. Chem., 2007, 2, 314-323 |





















