Current Research
I. Introduction
Research in the Chakraborty group focuses on the design of artificial biocatalysts (metalloenzymes) inspired by naturally occurring proteins, as applicable to bioenergy and conversion of feedstocks to usable chemicals. Metalloenzymes catalyze a wide range of criticial processes in biology with exceptional efficiency and selectivity. To circumvent the complexity of the native enzymes, we develop simpler functional model of these enzymes such that determinants for structure-function relationships and mechanistic outcomes can be elucidated. We employ two complimentary approaches in protein design, i.e. protein reenegineering and de novo protein design to create our artificial metalloenzymes (ArMs). Although simpler, the active sites are nestled within a protein framework which provides suitable dielectric environment while making the ArMs completely water soluble. Systematic modificiation of outer spehere residues allows one to probe how remote noncovalent interactions tune the function and mechanisms of the ArMs. We employ a suite of spectroscopic, structural, and analytical methods to characterize the ArMs and to determine their catalytic activity.
Students trained in our group will have strong foundations in interdisciplinary areas of protein design; inorganic chemistry; peptide chemistry; anaerobic techniques; UV-vis; CD; X-ray crystallography; liquid/gas chromatography; NMR; X-ray absorption spectroscopy; EPR; protein-film electrocatalysis; photocatalysis; transient absorption spectroscopy; and kinetics
II. Current Projects
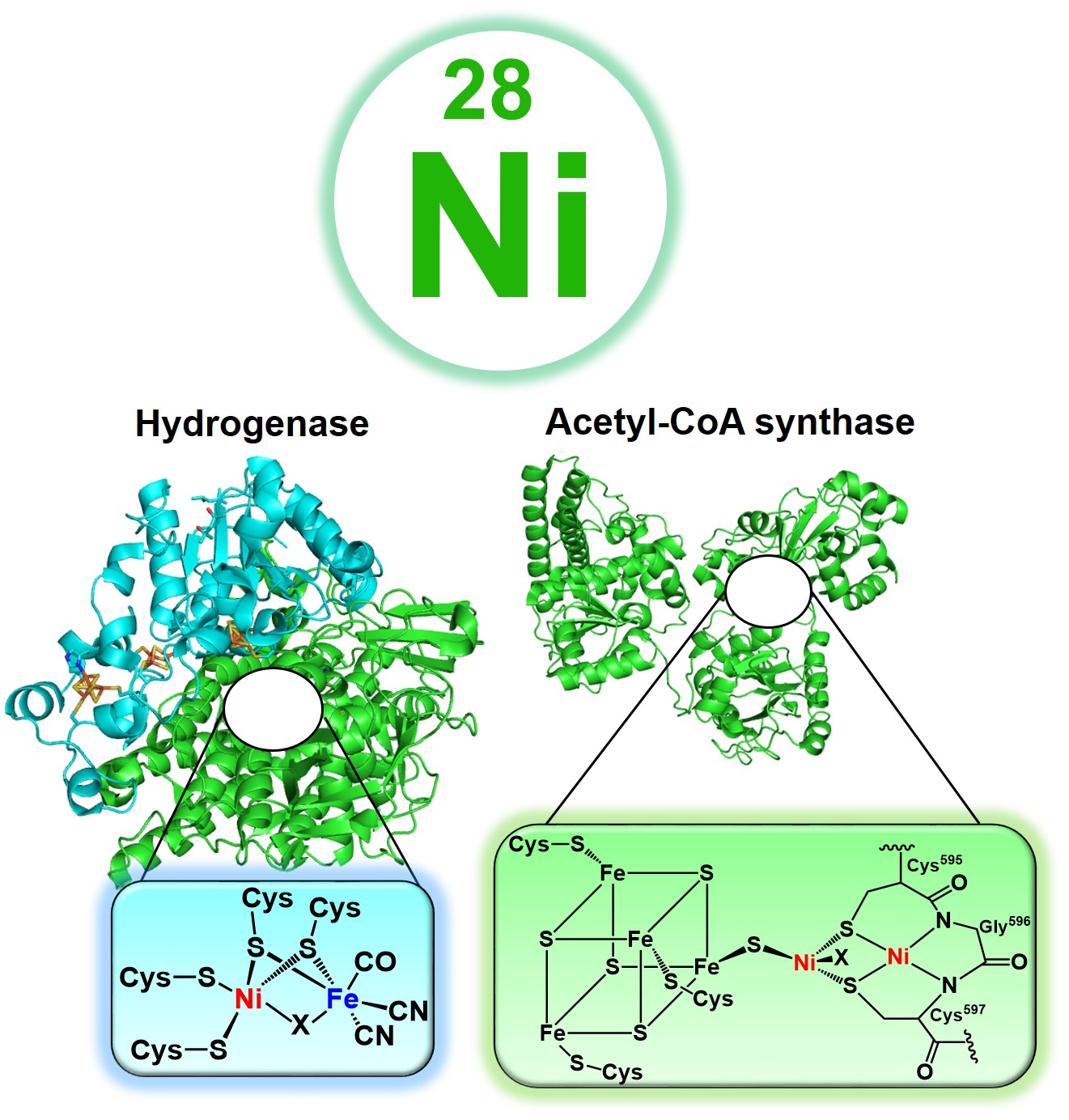
Artificial Ni-dependent metalloenzymes
Energy production from renewable resources in an environmentally benign way will mitigate our heavy dependence on fossil fuels and lower the carbon footprint by minimizing CO2 emission into the atmosphere. H2 is a potential green fuel source. However, as H2 is not available in abundance it must be produced in an earth-friendly manner. Hydrogenases catalyze one of the simplest chemical processes, i.e. combining protons and electrons to produce H2 gas while splitting H2 back to protons and electrons in a reversible manner at very low overpotentials. One approach to produce H2 is via artificial photosynthesis. We are developing biomolecular artificial hydrogenases (ArHs) employing protein design approach as functional analogs, which also provide a fundamental understanding of the various mechanistic aspects.
The global carbon cycle plays a central role in recycling CO2 and CO within the biosphere. Carbon monoxide dehydrogenase (CODH) and acetyl-CoA synthase (ACS) are tightly associated multimetallic enzymes in the Woodjungdahl pathway that coherently assimilate atmospheric CO2 into new C-C bonds of acetyl-CoA. The ACS activates CO and a methyl group at the A-cluster, known as the NiFeS cluster, to synthesize acetyl-CoA. ACS enzymes hold promise in making new chemicals from CO. We aim to develo artificial ACSs (ArACSs) using principles of protein design to circumvent the complexity of the native enzyme and attain a simplistic view of fundamental processes.

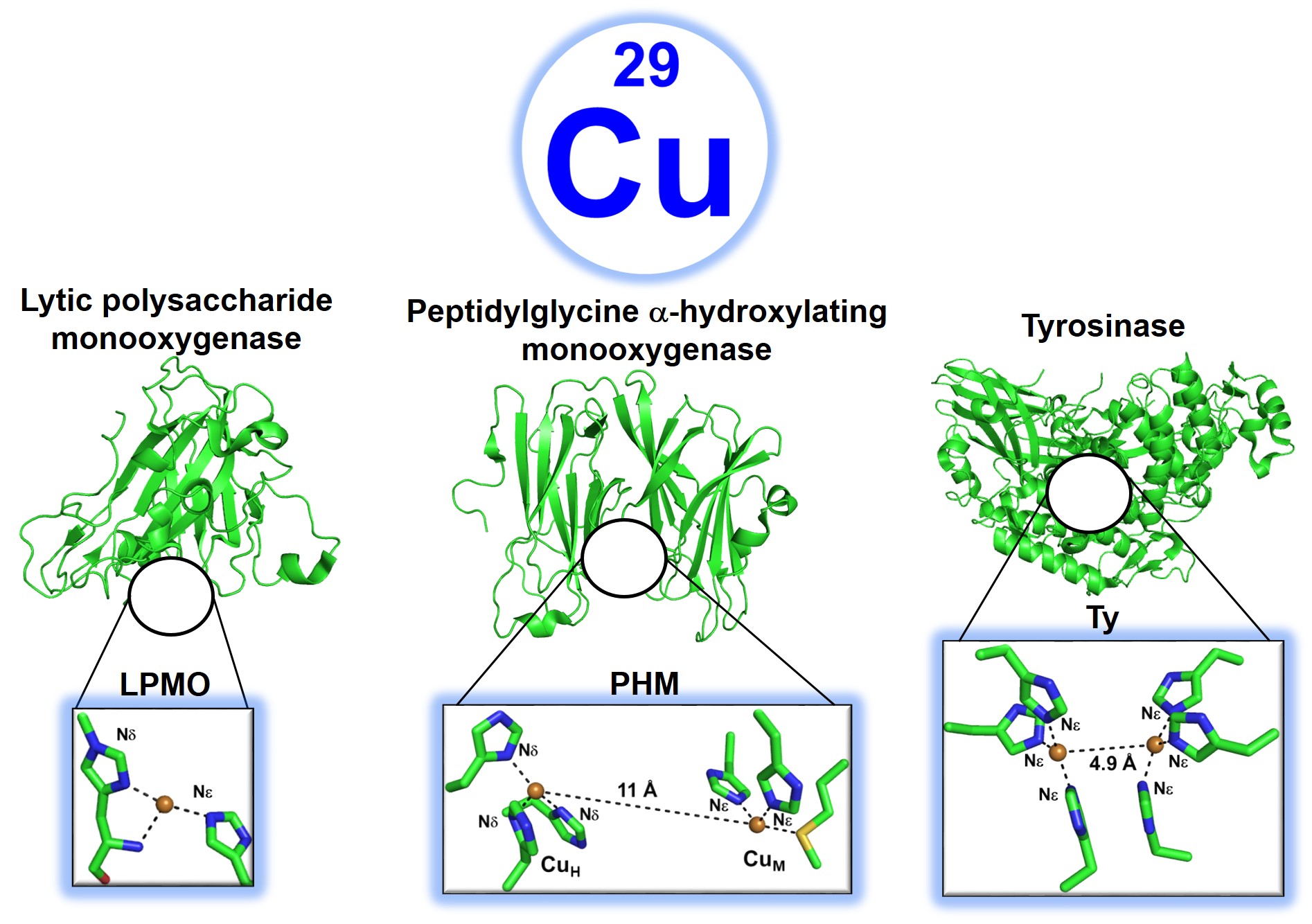
Biosynthetic proteins harnessing the oxidative chemistry of Cu enzymes
Cu, in contrast to Ni, is often involved in oxygenic biochemical processes, along with Fe. A number of Cu enzymes activate O2, H2O2, and C-H bonds with unprecedented efficiency. A few such enzymes are the mononuclear lytic polysaccharide monooxygenase (LPMO) which catalyzes the oxidative cleavage of glycosidic bonds in polysaccharides, the binuclear peptidyl alpha-hydroxylating monooxygenase (PHM) that hydroxylates C-H bonds of substrates at the CuM site with electron transfer (ET) from the CuH site ~11Angstorm away. Tyrosinase (Ty) is a coupled bninuclear Cu enzyme that hydroxylates monophenols to biphenols and o-biphenols to quinines. There are also multi-copper oxidases (MCOs) which reduce O2 to H2O by 4e-. Inspired by the structural and functional diversity of these unique classes of Cu enzymes, we are designing artificial Cu proteins (ArCuPs) that can activate O2/H2O2 and oxidize C-H bonds of small molecules by harnessing the oxidative power of Cu-oxygen intermediates. In addition to evolving the ArCuPs into efficient biocatalysts, we aim to determine the structure-function-mechanistic correlations of the underlying chemistry from our work.