Bioenergetics Phenotyping Core
BPC is the core facility in the School of Pharmacy that provides stakeholders within the Northern Mississippi region access to an XFe96 Seahorse Analyzer. This system is available with cold incubation to accommodate analyses of multiple species, including mammals, fish, drosophila, and bacteria.
Email:
keheath@olemiss.eduOffice Location:
Faser Hall 319Phone:
662-915-3096
- Home
- Departmental Directory
- Pharmacy
- Bioenergetics Phenotyping Core
Major Goals
The BPC aims to provide students, faculty, and research staff at the University of Mississippi (UM) and our partners within the Northern Mississippi region with access to a Seahorse XFe96 Analyzer for mitochondrial, cellular, and organismal analyses of bioenergetics and metabolism.
Funding Source
The Seahorse Analyzer was funded in part by the National Institutes of Health (NIH) under the Shared Instrumentation Grant (SIG) program (Award No.: S10OD026751)
Manufacturer's Details
Agilent is a global leader in life sciences, diagnostics and applied markets.Departmental Contacts
Fakhri Mahdi
- Principal R&D Biologist
Katie Heath
- Principal R&D Biologist
Services
Following certification, users operate the Seahorse Analyzer using their own materials. Contact Katie Heath to schedule usage time.
User Certification
Individuals must be certified by the Instrument Manager, Fakhri Mahdi, MS, before they may use the analyzer.User Training
Individuals may make a reservation by contacting Katie Heath, to schedule training with the Instrument Manager before certification.
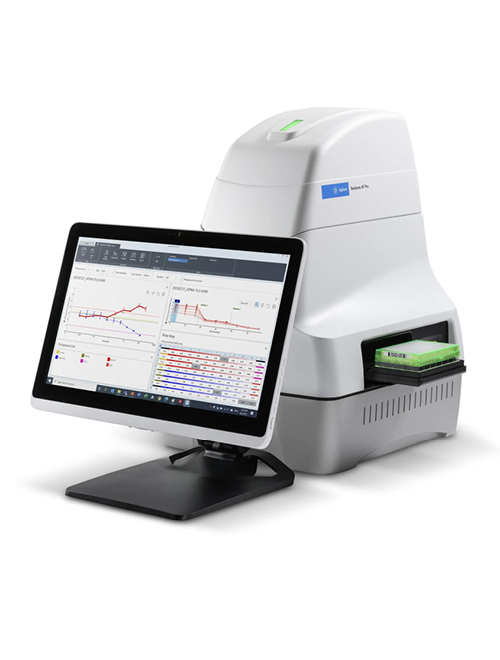
XFe96 Seahorse Analyzer
The Seahorse Analyzer simultaneously measures oxygen and pH in real-time to determine mitochondrial respiration (oxidative phosphorylation; OxPhos) via the oxygen consumption rate (OCR), and extracellular acidification rate (ECAR). Using these measurements, the total Proton Efflux Rate (PER) can be calculated as an accurate measurement of glycolysis rates.
These aspects of mitochondrial function and glycolysis are critical for cellular homeostasis and are perturbed in many disorders including aging, cancer, cardiovascular dysfunction, diabetes, obesity, metabolic disorders, neurodegeneration, and viral challenge.
Seahorse Analyzers are compatible with a variety of translational disease models, including primary cells and cell lines (adherent or suspension cells), isolated mitochondria, islets, 3-D spheroids, zebrafish embryos, C. elegans, D. melanogaster, yeast, tissue explants, and other biologicals.
- Seahorse XFe96 FluxPak (Agilent #102416-100)
- Contents: 18 XFe96 Sensor Cartridges, 20 XF96 V3 PS Tissue Culture Microplates
- Seahorse XFe96 FluxPak mini (Agilent #102601-100)
- Contents: 6 XFe96 Sensor Cartridges, 10 XF96 V3 PS Tissue Culture Microplates
- Seahorse XFe96 Spheroid FluxPak (Agilent #102905-100)
- Contents: 6 XFe96 Sensor Cartridges, 6 XFe96 Spheroid Culture Micoplates
- Seahorse XF96 V3 PS Culture Microplates (Agilent #101085-004)
- Contents: 10 Polystyrene plates
- Seahorse XFe96 Spheroid Plate (Agilent #102959-100)
- Contents: 1 Polystyrene plate. 1mm (divot diameter)
- Seahorse XFe96 Spheroid Microplates (Agilent #102978-100)
- Contents: 6 Polystyrene plates. 1mm (divot diameter)
- Media and Calibrant: Agilent Media and Clalibrant
- Plasma Membrane Permeabilizer (Agilent # 102504-100)
- Real-Time ATP Rate Assay Kit (Agilent #103592-100)
- ATP rates from OxPhos and glycolosis (via oligomycin, rotenone/antimycin A)
- Mitochondrial Stress Test Kit (Agilent #103015-100)
- ATP-linked respiration, proton leak, maximal respiration, spare respiratory capacity, and non-mitochondrial respiration (via oligomycin, FCCP, and rotenone/antimycin A)
- Glycolytic Rate Assay Kit (Agilent #103344-100)
- precise glycolysis and rapid metabolic switches not discernible in endpoint lactate assays (via rotenone/antimycin A and 2-dg)
- Energy Phenotype Test Kit (Agilent #103325-100)
- mitochondrial respiration, glycolysis, and rapid determination of metabolic phenotype/switching (via oligomycin and FCCP)
- Mitochondrial Fuel Flex Test Kit (Agilent #103260-100)
- dependency, capacity, and flexibility of cells to oxidize three major mitochondrial fuels: glucose (pyruvate), glutamine (glutamate), and Long-chain fatty acids, in basal energetic state (using inhibitors: UK5099, BPTES and Etomoxir)
- Mitochondrial Substrate Preference (Agilent #102720-100)
- Respiration and substrate preference in responses to stress (via palmitate-BSA fatty acid oxidation substrate).
- Glycolysis Stress Test Kit (Agilent #103020-100)
- basal glycolysis and glycolysis capacity upon blockage of mitochondrial ATP production using respiratory modulators (oligomycin, glucose, and 2-dg)
Our Rates
External (Non-UM) User Rate - $93 per hour
Our Policies
- Please, acknowledge the NIH Award Number in any publication resulting from the use of the Seahorse Analyzer (NIH award no.: S10OD026751). This gets reported to NIH annually as productivity on this award and supports the acquisition of future instrumentation grants to the University. As an example, the Acknowledgments section may include the following text: “Work was supported in part by the National Institutes of Health (S10 OD026751), the University of Mississippi (UM), and the UM School of Pharmacy.”
- All users are treated with equal respect, and all types of samples are given equal importance.
- All new users must email Katie Heath and provide the required details such as user information, sample details, account number, etc.
- To gain access to the Seahorse Analyzer, all users must have completed the Biosafety and Chemical Safety Training offered by UM Laboratory Services and must be certified for the use of the analyzer by the Instrument Manager, Fakhri Mahdi.
- Please do not change the instrument settings and never attempt to fix any equipment problem/s. In case of any problem/s, please contact the BPC manager, Katie Heath or Instrument Manager, Fakhri Mahdi as soon as possible.
- Users are responsible for their own data. Data and images should be transferred from the computer to portable media promptly.
- Do not download/install/upload any programs of files onto BPC computers. Do not delete/remove any data or images from the BPC computers.
- Following each session, the lab should be vacated promptly to allow other users to use the facility. The lab and equipment should be clean, operational, and in the standard configuration. The time required for clean up after a user and the time needed to correct any problems due to changes made in the configuration by a user may be billed to the user.
- Repair and opportunity costs for damage to instruments by the users may be billed to the user/Principal Investigator.
- Normal operating business hours are Monday to Friday, 8:00 AM to 5:00 PM. Approval for after-hours use is restricted to experienced users who have demonstrated a high degree of proficiency and independence and are at the discretion of the staff.
- Infectious samples are not approved for use with this instrument.
- All users must follow standard lab safety protocols. No food or drink is allowed in the facility at any time.
Investigators are required to recognize the role of the NIH Shared Instrumentation Grant in producing data for publication in the acknowledgment section of the manuscript. We ask that the authors submit a copy of the bibliographic reference or a PDF of the publication to the BPC manager Katie Heath for inclusion in the annual progress report.
Requested acknowledgment: “Work was supported in part by the National Institutes of Health (S10 OD026751), the University of Mississippi (UM), and the UM School of Pharmacy.”
