Safety Programs for Hazardous Materials and Equipment Management
Programs providing comprehensive guidelines, training, and oversight to ensure safe practices, regulatory compliance, and effective risk management.
Biological Safety
- Biosafety for Maintenance Personnel Training Handout - Bring to Training
- Safety Training Request Form
Contact Marlania Sharpe for any questions relating to training.
Know the Universal Biohazard Symbol!

- Biosafety means the safe work practices and proper use of personal protective equipment when handling biohazardous materials.
- Never Eat, Drink, or Smoke in a Biohazardous Lab.
- In a biohazard lab, just because a container or piece of equipment is not labeled, does not mean that it is clean. Always ask !
- Never move a piece of equipment unless you have asked. Never handle suspicious material. Always ask!
- Any piece of Equipment in a Biohazard area can be contaminated. This includes: Pumps, storage cabinets, freezers, waste containers, bags, or equipment used to transport or hold Biohazardous materials. Always ask!
- All gloves are not alike. Never touch yourself, especially your face or eyes, when wearing gloves. Always wash your hands after removing gloves.
- You must never clean up any materials that you spill in a lab. Contact you supervisor, someone in the lab, or the Physical Plant Base station – Control 1 on the Radio or 7087 by phone.
- If you forget any of this information, or if you ever have any questions, contact your supervisor or Health and Safety. Stop what you are doing until you are sure that it is safe. Never be afraid to ask questions.
- Typical Biohazardous materials used on the campus are: Blood, Body Fluids, Cells, Tissues, Infectious Microbes
- The purpose of containment and special work practices is to prevent accidental exposure.
- Exposure means the accidental transmission of a disease to a worker who enters an area to perform a service. Biohazardous materials cause infections.
- Accidental exposures most commonly occur through direct contact (touching) with: Broken or diseased Skin, the Eyes, the Nose and the Mouth.
- Contaminants can enter the body through: Puncture Wounds, Needle Sticks, Cuts or Abrasions
- Areas that use biohazardous materials will always be identified with the Biohazard symbol.
An Exposure control Plan tells you :
- Where biohazardous materials are used,
- What Jobs are at risk,
- Confinement procedures,
- Personal Work Practices,
- Protective Equipment to use,
- Where Immunizations are required
- (None are required for maintenance personnel since you are not expected to work with these materials), and,
- Medical Responses to accidents
The training you received is not everything you need to know. Your supervisor will update you periodically, lab personnel will tell of specific hazards in their areas.
Biohazard Levels on our campus :
- BL-1 Small risk
- BL-2 Moderate risk, primarily Labs that use blood or tissue (highest level we have)
- Most injuries are due to cuts, direct contact with materials and eye contact.
Biohazard Levels NOT on our campus:
- BL-3 Germs are cultured and they can be transmitted through the air
- BL-4 Exotic Areas
Basic Universal precautions are :
- Learn where biohazardous materials are used. Look for the symbol.
- Use common sense when working near these materials.
- Always wash your hands after leaving these areas.
- You will not be working with biohazardous materials, you need no special inoculation or shots.
- If you are on any medication and have to work in these areas, let your physician know. Let your supervisor know if your doctor says that it increases your exposure risks.
Report all accidents, no matter how small, even a scrape, if they occur in a biohazardous lab.
If others are working in a lab, use their protective clothing and gloves as a guide to what you should be using. You should always use the same type of protection as the people in the lab.
The University will always provide any protective equipment that you need to work safely in our job.
Barrier protection should be used at all times to prevent skin and mucous membrane contamination with blood, body fluids containing visible blood, or other body fluids (cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids, semen and vaginal secretions).
- Barrier protection should be used with ALL tissues.
- The type of barrier protection used should be appropriate for the type of procedures being performed and the type of exposure anticipated. Examples of barrier protection include disposable lab coats, gloves, and eye and face protection.
- Gloves are to be worn when there is potential for hand or skin contact with blood, other potentially infectious material, or items and surfaces contaminated with these materials.
- Wear face protection (face shield) during procedures that are likely to generate droplets of blood or body fluid to prevent exposure to mucous membranes of the mouth, nose and eyes.
- Wear protective body clothing (disposable laboratory coats (Tyvek)) when there is a potential for splashing of blood or body fluids.
- Wash hands or other skin surfaces thoroughly and immediately if contaminated with blood, body fluids containing visible blood, or other body fluids to which universal precautions apply.
- Wash hands immediately after gloves are removed.
- Avoid accidental injuries that can be caused by needles, scalpel blades, laboratory instruments, etc. when performing procedures, cleaning instruments, handling sharp instruments, and disposing of used needles, pipettes, etc.
- Used needles, disposable syringes, scalpel blades, and other sharp items are to be places in puncture resistant containers marked with a biohazard symbol for disposal.
- All specimens of blood and body fluids should be put in a well-constructed container with a secure lid to prevent leaking during transport.
- For routine procedures, such as histologic and pathologic studies or microbiologic culturing, a biological safety cabinet is not necessary. However, biological safety cabinets should be used whenever procedures are conducted that have a high potential for generating droplets. These include activities such as blending, sonicating, and vigorous mixing.
- Mechanical pipetting devices must be used for manipulating all liquids in the laboratory.
- Laboratory work surfaces must be decontaminated with an appropriate chemical germicide after a spill of blood or other body fluids and when work activities are completed.
- Contaminated materials used in the laboratory should be decontaminated before reprocessing or be placed in bags or other containers and disposed of according to Health & Safety procedures.
- Equipment that has been contaminated with blood or other body fluids must be decontaminated and cleaned before being repaired in the laboratory or transported to the manufacturer.
Autoclaves use superheated steam to sterilize materials and supplies for laboratory use and to prepare contaminated items for disposal. Because there are several brands and types of machines, it is the responsibility of the laboratory supervisor to properly train all of the personnel in the safe operation of the specific type of autoclave they are using. As a general rule the following table can be used as a guide for preparation and disposal. For specific techniques, more or less time, temperature, and pressure may be used as designated by the Principal Investigator (PI).
| Program | Temperature | Time | Drying Time | Items To be Sterilized |
| Liquids | 121°C (250°F) | 15 minutes | None | Distilled water, media solutions, other liquid prepared in closed bottles or flasks (not greater than 2 liters). Not for clinical applications |
| Unwrapped | 135°C (275°F) | 3 minutes | Default: 15 minutes, can be set from 1 to 99 minutes | Unwrapped instruments: metal glass and plastic objects. Heat resistant rubber tubing which will not be used in surgical applications. Any other items for which 135°C for 3 minutes is appropriate |
| Wrapped | 135°C (275°F) | 10 minutes | Default: 15 minutes, can be set from 1 to 99 minutes | Wrapped cloths, bandages, pads, fabrics, loosely wrapped individual instruments separated by fabric, wrapped trays of loose instruments, instruments in bags, heat-resistant rubber tubing. Any other items for which 135°C for 10 minutes is appropriate. |
| Packs | 121°C (250°F) | 30 minutes | Default: 15 minutes, can be set from 1 to 99 minutes | Packs of towels. Groups of instruments in commercially prepared packs. Instruments subject to prolonged storage. Surgical gloves wrapped for sterilization. Any other items for which 121°C for 30 minutes is appropriate, except liquids |
| Optional | User defined from 100°C to 136°C (212°F to 277°F) | User defined | User defined 1 to 99 minutes | Items appropriate to the user defined parameters. Dental handpieces should be run in this cycle at 132°C for 10 minutes. |
When using any type of autoclave, a few general rules must always be followed to insure its safe operation.
- Never attempt to open the door while the machine is operating.
- Always check the jacket pressure gauge to make sure that it is reading 0 PSI before opening the door.
- Do not overfill containers when autoclaving liquids.
- Use a catch basin to capture overflow when autoclaving any materials.
- Periodically check chamber trap and clean as necessary.
- When preparing materials for disposal, do not autoclave more than can easily be placed in an appropriate disposal container.
- If the machine is not operating properly, notify the laboratory supervisor. Do Not Attempt To Make Repairs. This should be done only by a trained technician.
Laboratory Services tests all operational autoclaves biannually and as needed after major repairs to ensure proper operation.
- Several cuts or lacerations due to the improper disposal of laboratory glassware have recently been reported on the campus. In an effort to eliminate or minimize these injuries, certain procedures will be followed when discarding broken or unserviceable glassware.
- Broken glassware should immediately be cleaned up. A laboratory should already have a small brush and dust pan available to clean up after small accidents. Forceps or duct tape can be used to pick up the smaller pieces of broken glass.
- Discarded glassware must not contain any hazardous wastes, Medical Waste, Pathological Waste or Radiological Wastes.
- If the glassware contains hazardous wastes or Radiological Wastes, please call Laboratory Services (5433) for disposal instructions.
- If the glassware contains or is contaminated with any pathological or biological materials, refer to the Sharps and Biological Waste Disposal Flow Chart (PDF file) or call Laboratory Services (5433) for disposal instructions.
What you need to know:
- Discarded glassware must be placed in a small puncture proof, double-lined cardboard box or a container specifically designed for the disposal of glassware
- The box must be securely sealed with tape.
- Any cardboard box may be used, provided it is sturdy and of a size that will not weigh more than 40 pounds when full.
- The container must be labeled as to the contents.
- CAUTION LABORATORY GLASSWARE ONLY!
- NO HAZARDOUS WASTE, MEDICAL WASTE, PATHOLOGICAL WASTE OR RADIOLOGICAL WASTES
- YOU MUST PLACE THE CONTAINER DIRECTLY INTO THE DUMPSTER.
- Never allow Custodial Services to handle broken glassware.
- Never use laboratory glassware boxes for the disposal of
- Sharps
- Biohazardous materials
- Liquid wastes
- Chemically contaminated laboratory glassware/plastics or plastic-ware
- Chemical containers that can’t be disposed of as regular solid waste
Laboratory glass that is disposed of in cardboard boxes must be appropriately decontaminated, where necessary, prior to disposal. If you have any questions about this disposal procedure, please contact Laboratory Services (5433) for additional information or instructions.
Empty Containers
Empty containers of five (5) gallons or less may be placed in dumpster's if they meet the definition of the Resource Conservation Recovery Act (RCRA) empty container rule.
- Any container that previously held a hazardous chemical or waste is defined as empty if:
- No hazardous materials can be poured, pumped or drained from the container, AND,
- No hazardous materials remain in the container that can be feasibly removed, AND,
- the walls of the container must not contain any significant residual materials, AND,
- The label is removed or defaced, AND,
- The lid is removed, AND,
- The container is placed directly into a dumpster.
Triple-rinsing is not required to comply with the RCRA empty container definition.
- Laboratory gloves worn in chemical, biological or radiological work areas must be removed before leaving the laboratory setting.
- Within the laboratory, gloves are used to prevent contamination between hazardous materials and individuals. Improper use of gloves outside of the laboratory setting may become a source of contamination to others. Even “clean” gloves can cause uneasiness and apprehension in others when worn outside of the work area.
- Gloves should not remain on your hands when exiting the work area (including crossing the hallways), when answering the telephone, when opening or using uncontaminated equipment, or when touching door handles. Contaminated materials should not be removed from the laboratory without overpacking the container. This simple procedure will reduce the potential for accidental exposures and eliminate the need for gloves when traveling between laboratories.
Custodial Services are responsible for the cleanliness of the University facilities, including Offices, Classrooms, Meeting Rooms, Hallways and Restrooms. Other areas of the University usually require advanced technical training before personnel can fully understand and adequately protect themselves from the hazards within these areas. Since custodial staff are neither trained nor assigned to work in these areas, they will not provide unsupervised services in these areas. Laboratory personnel will be required to take care of their own housekeeping.
Specifically, custodians will provide no routine cleaning services within :
- Laboratories,
- Shops, Storage Rooms,
- Areas designated by the Animal Care and Use Committee,
- Other areas designated by the Superintendent of Custodial Services, the Fire Inspector, or the Research and Environmental Compliance Officer.
The following policy will protect the custodial staff while allowing for limited services to hazardous locations :
- No trash of any kind will be removed from inside any hazardous areas, including research laboratories, by custodial staff. If you wish to have your trash taken away, you must collect it from inside your lab and place it in a designated waste container just inside your lab door, or in an designated area outside your lab. Custodial Staff will specify collection areas for each lab or hazardous location.
- The waste cans to be used will be supplied. Waste cans containing trash from research labs will use trash liners of a distinguishing color to be supplied by the custodial staff.
- No glass bottles, i.e., solvent or empty chemical bottles, or sharps are to be placed in this trash. Broken glass must be placed in standard box containers. When these boxes are full, they must be sealed with tape and placed in the dumpster behind the building by the lab staff. bottles.
- Sharps are to be collected in special containers designed for this purpose. Contact Shane Kesler for specifications and guidelines. Full containers will be collected by Laboratory Services.
- When custodial staff need access to a hazardous area for non-emergency cleaning (end of the semester, etc.) the Area Supervisor will :
- provide continuous, Authorized Supervision while custodial personnel are in the area.
- assure that all hazardous materials are capped, sealed and stored,
- have all hazardous equipment powered down, and,
- cease all hazardous operations.
- Established procedures for the disposal of Hazardous Materials (including waste solvents, solid chemicals, biohazards, radioactive materials, etc.) are not affected by this policy.
Purpose
These procedures will assist personnel in safely removing all hazardous substances from a laboratory area and ensure that an area is free from hazardous contamination.
These guidelines apply to ALL laboratories, darkrooms, stockrooms, shops, research/teaching/maintenance facilities, and any other area where hazardous materials are used or stored.
As the Principal Investigator, it is the Supervisor / Faculty Member’s responsibility to ensure that all areas they supervise are properly decontaminated before the research staff vacates.
Biological and Pathological Materials
- Disinfect work surfaces that may be contaminated with biological agents.
- Autoclave and dispose of unwanted biological agents in appropriate containers.
- All sharps (needles, pipets, lancets, broken glass, etc.) must be placed in puncture proof containers for disposal.
- All waste must be clearly labeled and Laboratory Services (5433) must be contacted for removal.
Hazardous Materials and Chemicals
- All chemicals must be labeled and sealed.
- All waste must be cleearly labeled and Laboratory Services (5433) must be contacted for removal.
- Wipe all surfaces and equipment with a warm solution of soap and water. This includes fume hoods, bench tops, refrigerators, sinks and floors.
Radioactive Materials and Radiation Generating Devices
- A wipe test must be done on all surfaces and equipment where radioactive materials have been used. Please submit this data to Laboratory Services.
- Radioactive waste must be properly packaged. Contact Laboratory Services to request disposal of Radioactive Materials.
- Remove all “Radioactive” labeling and signs from equipment once it is decontaminated.
The lab will be certified as Declassified by Laboratory Services when :
- All Lab equipment has been decontaminated and surveyed,
- All Radioactive materials are removed and secured, and
- All survey results are accepted and approved by Laboratory Services.
Chemical Safety Resources
During routine servicing and repair or dismantling of a chemical fume hood the potential exists for exposure to hazardous substances that had been used or stored in the hood. To guard against this, certain protective measures, appropriate to the specific situation, should be implemented before the work begins.
I. Before a fume hood is serviced or dismantled Lab personnel must:
- Remove all equipment in the hood, which may impede or impair access.
- Remove all chemicals and radioactive materials in the hood, which may pose a hazard.
- If necessary, decontaminate the interior of the hood as appropriate.
- If necessary, don PPE (Personal Protective Equipment: such as goggles, respirator, gloves, coveralls or arm guards).
- If the hood fume needs to be turned off, notify laboratory workers and post an “Attention, This Hood is Being Repaired” Notice on the hood.
Note : The iodination mini hood housed within a fume hood should be shut down and covered with plastic whenever the fume hood requires servicing, this includes servicing at the roof.
A designated person from the laboratory is responsible for ensuring that the procedures mentioned above have been done. Upon completion of the necessary decontamination procedures, the responsible party must notify the person listed at the bottom of the Hood Repair Notice so that repair work may begin.
The DHS may be consulted regarding appropriate decontamination procedure.
II. Fume Hood Service Procedure
The following procedures are to be followed by anyone who must service any part of a fume hood system at the University. (Service includes mechanical work, sheet metal work, painting, and electrical work.)
- Locate the appropriate fan or motor on the roof that needs service and the room in which the fume hood is housed. Electricians should have hood-to-fan information. A specific fume hood and the room in which it is housed can be identified by either a fan number marked on the fume hood structure or a room number marked on the fan housing.
- Communicate to lab personnel the need to service the fan or hood and obtain permission to shut down the hood. If lab personnel are not available, contact the building manager or Department Chair to obtain permission to shut down the hood.
- Do Not Turn Off Fan Without Permission From An Authorized Person and after all hazardous materials have been removed from the fume hood.
- Fill out a Hood Repair Notice and fix it to the hood sash. Then shut down the fan. Note: Information on the tag should include:
- Date of shutdown
- Building location
- Room number
- Estimated time period when hood will be off
- Your name or your supervisor’s name and phone number
- If the fan to be worked on is located near hood exhaust stacks, which do not have a 7 to 10 ft. extension, those fans must also be turned off. If this is not possible, an appropriate respirator and safety glasses supplied by your supervisor are to be used (consult DHS at 5433).
- After service is completed, restart the fan and remove the Hood Repair Notice from the fume hoods.
- Safety Training Request From
- Chemical Safety Training Handout. - Bring to Training
The requirements for testing and initial certification of new campus hoods are described above. Existing hoods are required to have their performance tested and certified on an annual basis.
- DHS is responsible for performing the annual testing and certification of campus fume hood performance to meet campus requirements. DHS is responsible for maintaining testing and certification records.
- Once a hood has been certified, DHS will attach a small inspection tag. This tag will list the class, average face velocity and date of testing. Each hood will also be provided with a green sticker, which contains helpful reminders concerning the use of your hood and who to contact in an emergency.
- If hood performance is significantly outside the limits of acceptable performance, DHS will post the hood as Out of Service for continued use and inform lab personnel. The Department in charge of the lab submits the work order for the repair of a hood. After Physical Plant has performed repairs or adjustments to the hood, DHS will retest and certify the hood.
- If you have a fume hood that has not been inspected in the past year, or still has an Out of Service notice and has not been repaired, please contact the DHS office (5433).
USE YOUR HOOD WISELY! Help Prevent Air Pollution – Minimize use of volatile chemicals. Keep containers properly labeled and closed. Do not evaporate hazardous waste or other volatile chemicals. Obey Fume Hood Repair signs.
For your safety : Use this hood to reduce your exposure to airborne hazardous chemicals. Avoid storing chemicals here. Do not place items in the hood that will obstruct air flow.
To report a spill that you can not clean up yourself, call Health and Safety 915-5433.
For hazardous waste disposal, call Health and Safety 915-5433.
For fume hood maintenance, have your department put in a notification to the electric shop.
Chemical Fume Safety Resources & FAQs
The following safety procedures are recommended for any personnel performing maintenance on laboratory fume hoods and its exhaust components.
- Assume that the duct lining, fume hood lining and all internal exhaust components are potentially contaminated with chemical residues.
- Generally, maintenance personnel will not be working with the internal hood components.
- If potential contamination is suspected, maintenance personnel must contact the Department of Health & Safety (DHS) (915-5433) for assistance.
- The Lab Supervisor or Department Chair must be contacted and informed before maintenance is performed on their fume hood.
- The Lab workers will need time to contain or remove all hazardous materials from the fume hood.
- If work is to be performed on the portion of the fume hood within the lab, it may need to be decontaminated before work can begin.
- If radioactive materials are used in the hood, the maintenance personnel may request that DHS (915-5433) perform a survey of the hood before maintenance work begins.
- On the day the work is to be performed, maintenance personnel must post the hood with the red “Attention–This Hood Is Being Repaired” notice and fill in the appropriate information.
- It is important that this notice is easily visible by all lab workers that may use the fume hood.
- Nitrile gloves, eye protection and chemical resistant coveralls (Tyvek™) may be necessary if contact with internal components is required.
- When working around sharp objects like sheet metal, wear appropriate outer work gloves in addition to inner Nitrile gloves.
- Eye protection is especially important when working on systems, which are in operation.
- Special care must be used when working on energized systems.
- Prior approval of your supervisor and special procedures may be needed before working on energized systems.
- Since other exhaust systems from the building may be operational, a chemical cartridge respirator should be worn during all roof top maintenance.
- Note: Before respirators are to be used, you must determine which type of respirator is needed and how it should be properly fitted.
- For help in respirator selection and use, please call DHS (915-5433). Workers should always wear protective eye wear (goggles) when working near operational hood exhaust vents.
- After the work is completed, the Nitrile gloves should be discarded and hands should be washed with soap and water.
- The Red Hood Repair Notice should be removed from the fume hood after the hood has been certified it is operating effectively.
- If any assistance is needed in reviewing the hazards present in any fume hood, please call the Department of Health & Safety (DHS) at 915-5433.
Health and Safety calibrates all chemical and radiological fume hoods on the main campus, as well as those located at the Biological Field Station, to ensure that they are operating within the parameters recommended for the types of hazardous materials used. Calibration is the measurement and determination of both the minimum and the total airflow capabilities of the fume hood. Based upon the measurements, the performance of the hood is rated based upon the following generally accepted criteria:
| Class 0: Average minimum flow rate of less than 75 feet per minute (FPM). This hood is unsafe for use with hazardous chemicals and radioactive materials. It is acceptable ONLY for nonhazardous materials and nuisance odors. |
| Class 1: Average flow rate of between 75 and 100 FPM. This hood is unsafe for use with hazardous chemicals and radioactive materials. It is acceptable ONLY for nonhazardous materials, nuisance odors, and heat dissipation, |
| Class 2: Average flow rate of at least 100 FPM, and no single point less than 75 FPM. This hood is appropriate for general laboratory usage and most low level radioactive materials. |
| Class 3: Average flow rate of 150 FPM. This hood is usually recommended or required when working with materials that have moderately toxic or radioactive characteristics. |
| Class 4: Average flow rate greater than 200 FPM. This hood is required when working with highly toxic and highly radioactive materials. |
Specific criteria for checking a hood is based upon the design features as well as the size of the hood. Generalized testing points for several sizes and configurations are indicated by diagrams below.
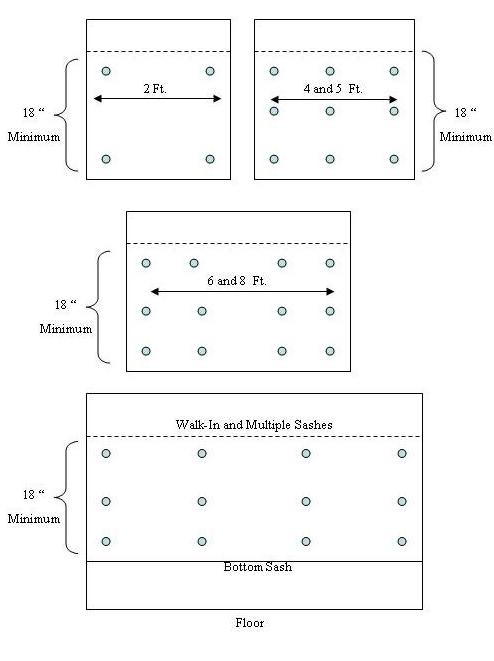
All hoods with movable a sash are tested with a minimum opening of 18″.
Hoods with a 2 ft. wide sash and a constant flow motor are checked with a digital anemometer at 4 points-2″ from the top and both sides, and 2″ from the bottom and both sides.
Hoods with 4 and 5 ft widths and constant flow motors are checked with a digital anemometer at 9 points-2″ from the top and both sides, 2″ from the bottom and both sides, halfway from top to bottom 2″ from both sides, and in the center at the top, middle, and bottom.
Hoods with 6 and 8 ft wide sashes and constant flow motors are checked with a digital anemometer at 12 points-2″ from the top and both sides, 2″ from the bottom and both sides, halfway from top to bottom 2″ from both sides, 1/3 of the distance from each side at a point 2″ from the top, 2″ from the bottom, and halfway from top to bottom at each 1/3 distance.
Hoods with variable flow motors are checked the same as those above, but the height is set at the manufacturer’s installed stops.
Hoods with split sashes have both sashes set at the stops.
Walk in hoods are checked with the bottom sash completely closed, and the top sash set to the standard height, and checked at the corresponding width of the hood.
Walk in hoods with multiple doors are checked with all bottom sashes and all but one upper sash down. The open sash is set to the proper height and checked at the corresponding width locations.
During calibration of the hood, the operator will record the unique hood ID number, the test date, measured flow rate from each test point and the calculated Class on an inspection report sheet. An identification label is dated and affixed to the hood faceplate, including a unique hood ID number, inspection date and Class. Calibration testing information, including recorded flow test rates, is entered into a Departmental database. The original records are filed in accordance with the Departmental Records Retention Policy.
The Electric Shop of the University of Mississippi Physical Plant Department performs all fume hood maintenance. Your department needs to submit a notification with the Electric Shop to have the hood maintenance performed.
If your fume hood has an Airflow Indicator, check it to see if it operating in the normal range. If the Airflow Indicator shows that the hood is not working properly or you feel the indicator is incorrect, call DHS (5433). If your hood does not have an airflow indicator, call DHS (5433).
Construction, Installation, and Renovation
Laboratories with fume hoods must be designed to have no recirculation of air to that lab or any other spaces. Recirculation of laboratory air will result in indoor air quality problems.
Windows in labs containing fume hoods must be fixed closed. Breezes coming in through open lab windows can adversely affect the proper functioning of a hood. Turbulence caused by these wind currents can easily bring the contaminated air inside the hood out into the lab.
Supply air intake locations must be located a minimum of 50 feet from the discharge point of any fume hood. Intakes should also not be located in the vicinity of loading docks, generators or other devices generating harmful emissions. Re-entrainment of emissions will cause obvious indoor air quality problems.
Noise from fans, ductwork and air velocities shall not exceed 65 dBA inside the lab area.
Minimum 18 gauge, Type 316 stainless steel. Coated galvanized steel may be considered for non-hospital installations under special conditions. However, special attention to quality control is required.
- Heliarc inert gas with Type 316 welded seams.
- Follow Sheet Metal and Air Conditioning Contractors’ National Association (SMACNA) Round Industrial Duct Construction Standards for duct supports and reinforcement using stainless steel material.
- Follow SMACNA 1985 HVAC Duct Construction Standards using Type 316 stainless steel for exhaust stack on roof.
- E. New duct installations must be tested at negative pressure, 1 1/2 times its operating pressure (per SMACNA). Test should show zero leakage.
Duct velocities should be maintained below 1,600 feet per minute (fpm) to minimize noise.
Fans shall be constructed of materials compatible with the chemicals being transported in the air through the fan. If flammable gas, vapor or combustible dust is present in concentrations above 20% of the Lower Explosive Limit (LEL), the fan construction shall be as recommended by AMCA’s Classification for Spark Resistant Construction.
Exhaust stacks should be secured with earthquake restraints, where applicable.
Waste outlet should be connected into the building’s acid resistant laboratory waste system, if present.
Lighting fixtures should be of the fluorescent type. Fluorescent bulbs give off less heat than conventional bulbs. They help maintain a safe and comfortable work area inside the hood.
Light fixtures should be sealed and vapor tight, UL-listed, and protected by a transparent impact resistant shield. The potential for flammable or combustible atmospheres requires explosion-proof electrical equipment.
Sashes may be horizontal, vertical, or a combination, and should have the capability to completely close off the hood face.
Laminated safety glass, when internal temperature is anticipated to be less than 160°F.
- Tempered safety glass when high internal temperatures are anticipated that will result in sash surface temperatures greater than 160°F.
Laser Safety Resources
Below are forms and instructions related to laser safety:
Laser Safety training is provided through CITI training. Conctact RSC at rsc@olemiss.edu for more information.
Radiation Safety
Below are resources for radiation safety regulations:
Radiation Safety Training is provided through CITI training. Contact RSC at rsc@olemiss.edu for more information.
- The Radioactive Material Purchase Application form (DHS-026) must be completed before it is sent to Laboratory Services or the order may be delayed or canceled.
- Purchases of $5000 or less only require one written quote from a vendor for the material(s) to be purchased. Purchases exceeding $5000 require two quotes.
- Quotes must be signed by a sales representative or an original email must accompany the Application form.
- Quotes should only include the material(s) listed on the Application form. Quantities and material(s) should be listed as stated on the quote. Quotes submitted with material(s) not included on the Application form will be returned.
- The form must (be):Dated and signed by the Principal Investigator
- Include the Cost Center / Account Number
- Signed by the Signatory Officer
- Delivered (mail, FAX, E-mail) to Laboratory Services for review and processing
Purchase, Receipt and Delivery of Radioactive Materials
- Completed application forms will be processed into a purchase requisition.
- The materials will be ordered by Procurement Services.
- All Radioactive material(s) are initially delivered to Laboratory Services.
- All packages received will be subjected to the following upon receipt:
- Visual Inspection
- Checked for leakage
- Surveyed for removable contamination (as applicable)
- The material will be added to the University Inventory
- Laboratory Services will deliver the material and the associated paperwork to the authorized location of use, properly store the material, and notify the intended recipient.
Notify Procurement to process payment for the materials
- Following the satisfactory receipt of the material, Receiving will be notified at receiving@olemiss.edu to process a GOODS RECEIPT for the Purchase Order.
- Approved Users requesting to work with hydrogen-3 (tritium) in milliCurie or greater quantities, as well as other personnel in the immediate area, must take special care to prevent the release of tritium to the environment and to prevent its accidental ingestion or inhalation.
- All personnel working within the approved lab must first submit a preliminary background bioassay and an individual’s urine must be evaluated for tritium content, no earlier than 24 hours and no later than 72 hours, after the use or potential exposure to any tritium levels meeting or exceeding those given in Table 1.1
Table 1.1 When is a Bioassay Required?
A tritium (H-3) bioassay is required, when any of the activity levels or concentrations given in the table below are met or exceeded.
| Processes | HTO (and forms Other than those in the right-hand column) HTO=tritiated H2O | HT or T2 Gas(contained in sealed process vessels) | Nucleotide Precursors | HTO (mixed with more than kg of inert H2O or other inert substances) |
| In open room or bench, (with possible escape of tritium from process vessels) | 0.1 Curies (3.7 GBq) | 100 Curies (3700 GBq) | 0.01Curies (370 MBq) | 0.01Curies (370 MBq) |
| With possible escape of tritium, (carried out within a fume hood of adequate design, face velocity, and performance reliability) | 1 Curie (37 GBq) | 1000 Curies (37,000 GBq) | 0.1 Curies (3.7 GBq) | 0.1 Curies (3.7 GBq) |
| Carried out within gloveboxes, (ordinarily closed, but with possible release of tritium from process and occasional exposure to contaminated box and box leakage) | 10 Curies (370 GBq) | 10,000 Curies (370 TBq) | 1 Curie (37 GBq) | 1 Curie (37 GBq) |
How much Tritium is allowed to be present in my body? The Annual Limit on Intake (ALI) by ingestion or inhalation of tritium (H-3) is: 3 x 10E9 Bq (~80 mCi) of tritiated water. (reference: ICRP 30)
Table 1.2 Uptake limit concentration of hydrogen-3 in urine.
| Criteria | Limit |
| Weekly Limit for Whole Body Uptake | 2 x 10-3 µCi / ml |
| Permit Holder Investigation Level | 6 x 10-4 µCi / ml |
| Radiation Safety Notification Level | 2 x 10-4 µCi / ml |
General Precautions
- All procedures involving milliCurie quantities of tritium (where the tritium compound is or could become volatile) must be conducted in a properly operating chemical fume hood.
- This includes:
- Opening of packages;
- Dilution of stock solutions; and
- Chemical procedures.
Individuals handling tritium compounds shall wear a lab coat and protective gloves.
How do I perform a Tritium Bioassay?
- Each individual requiring a bioassay shall collect, handle, and count their own samples.
- If an LSC is not available, you may submit a sample and a partially completed report to DHS.
- This shall be done by collecting all of the urine in a single urination-void, withdrawing a 1-10 ml sample (5 ml preferred) and discarding the remainder.
Sample Preparation
- No specific sample preparation is required.
- Low potassium glass or plastic vials should be used to minimize background counts.
- An adequate amount of scintillation cocktail should be added and the sample should be shaken well.
- The sample vials should be allowed to dark adapt in the scintillation counter for one hour prior to counting to minimize fluorescence.
Why can’t I use a Geiger Counter?
- Portable survey instruments are inappropriate for monitoring the weak (18.6 keV max.) beta particle emitted by hydrogen-3.
- A liquid scintillation counter is adequate for counting contamination wipes and bioassay samples. This counter should be calibrated on an annual basis using National Institute of Standards and Technology (NIST) traceable standards.
How do I calculate the tritium concentration?
Formula for Determining Concentration
- The concentration of tritium in the sample in units of µCi / ml can be calculated using the following formula:
Concentration = [ Cs – Cb ]/ [ ( 2.22 x106 ) E V ] where: Cs = Gross sample counts per minute
Cb = Background counts per minute
E = Counting efficiency for tritium
V = Volume of sample
2.22×106 = DPM per µCi
Formula for Determining Minimum Detectable Activity (MDA)
- The minimum detectable activity for the counter in units of µCi / ml can be calculated using the following formula:
MDA = [ 2.71 + 4.65(Rb T)1/2 ] / [ ( 2.22 x106 ) E V T ] where:Rb = Background count rate
T = Counting time
E = Counting efficiency for tritium
V = Volume of sample
2.22×106 = DPM per µCi
* The calculated MDA must be lower than the limits on uptake or the effluent limits or the counting procedure is not valid.
Records Retention
- Use the Tritium Bioassay Worksheet (DHS-28) to comply with records retention requirements.
*The original copy of Form DHS-28 must be submitted to Laboratory Services.
Using Program Forms and Worksheets
- The forms and worksheets used with his document provide the basis for an individual to document the surveys and assessments performed.
- All of the worksheets describe the policy that must be met for radiation safety surveys.
- Form DHS-28 includes the minimum amount of information necessary to provide traceability and repeatability of the measurements, if needed.
Below are required posters to have in areas with radiation use:
